- Expert Authors

- Conferences
- Publications

NeurologyLive® Year in Review 2024: Top Stories in Multiple Sclerosis
Key takeaways.
- Revised McDonald criteria enhance MS diagnosis specificity with new biomarkers, expanding diagnostic capabilities.
- Ublituximab shows promise in transitioning from anti-CD20 therapy, demonstrating tolerability in the ENHANCE trial.
- Ozanimod maintains long-term efficacy in relapsing MS, as shown in the DAYBREAK trial.
- High burnout rates among MS physicians highlight the need for systemic changes to support quality care.
- Tolebrutinib significantly slows disability progression in non-relapsing secondary progressive MS, addressing a large unmet need.
As part of NeurologyLive®'s Year in Review, take a look at our most-read news in multiple sclerosis in 2024.
In 2024, the NeurologyLive ® staff was a busy bunch, covering clinical news and data readouts from around the world across a number of key neurology subspecialty areas. From major study publications and FDA decisions to societal conference sessions and expert interviews, the team spent all year bringing the latest information to the website's front page.
Over the past 12 months, there have been several significant advances in the field of multiple sclerosis (MS), including the first approved biosimilar to treat patients with relapsing forms of the disease. The improvements to therapeutics, powered by an increased effort from industry leaders, has expanded the ways in which clinicians can personalize treatments for patients with MS. With the amount of ongoing research, it's nearly impossible to narrow down just 10 stories that have impacted the MS field this year.
Scroll below as we highlight some of the most-read MS content on NeurologyLive ® this year. Click the buttons to read further into these stories.
1. Revised McDonald Diagnostic Criteria Signals New Era in Multiple Sclerosis Treatment
The 2024 revisions presented at the 40th Congress of the European Committee for the Treatment and Research in Multiple Sclerosis mark a pivotal shift toward increased specificity through the addition of pathologically-specific biomarkers and expanding who can be diagnosed with MS. The 2024 revisions include some of the most substantial and paradigm-changing features since the inception of MS diagnostic criteria in 2001.

2. Switching From Anti-CD20 Therapy to Ublituximab Shows Promising Results in Phase 3b ENHANCE Trial
New interim data from the phase 3b ENHANCE trial (NCT05877963) of patients with multiple sclerosis (MS)demonstrated the tolerability of transitioning from treatment with intravenous (IV) anti-CD20 to ublituximab (Briumvi; TG Therapeutics), a novel monoclonal antibody approved therapy for relapsing forms of MS. These results suggest the successful transition of switching from anti-CD20 therapy to ublituximab for this patient population even with elimination of the starting dose.

3. Phase 3 DAYBREAK Trial Highlights Long-Term Efficacy of Ozanimod for Relapsing Multiple Sclerosis
New long-term data from the phase 3 open-label extension DAYBREAK trial (NCT02576717) presented at the 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum , February 29 to March 2, in West Palm Beach, Florida, showed sustained efficacy for measures of disease activity and progression with ozanimod (Zeposia; BMS), an FDA approved disease-modifying therapy for patients with relapsing forms of multiple sclerosis (RMS).

4. FDA Approves Single-Dose SelfJect Injector Delivery Device for Inflammatory and Autoimmune Conditions
The FDA has approved a new single-dose, pre-filled delivery device, named SeflJect, to be used for Acthar Gel (repository corticotropin injection; Mallinckrodt), a treatment indicated for a various number of chronic and acute inflammatory and autoimmune conditions, including multiple sclerosis (MS). SelfJect is intended to provide an appropriate subcutaneous dose of the therapy and may help improve patients' control of administration.

5. Nasal Foralumab Shows Attenuation of Microglial Activation, Clinical Stabilization in Non-Active Secondary Progressive MS With PIRA
Significant findings from an open-label expanded-access program revealed that treatment with investigational foralumab (Tiziana Life Sciences) resulted in damped microglial activation and clinical stability in patients with non-active secondary progressive multiple sclerosis (na-SPMS) who had progression independent of relapses (PIRA). Investigators are planning a double-blind, placebo-controlled, dose-ranging study of the agent in this population using (F-18)PBR06-PET, the main tool used to measure microglial activation, as a primary end point.

6. Survey Reveals High Burnout Rates Among Physicians in Multiple Sclerosis Across the United States
New findings from a small sample survey presented at the 2024 Consortium of Multiple Sclerosis Centers (CMSC) Annual Meeting , held May 29 to June 2, revealed high rates of burnout and job stress among physicians treating patients with MS in the United States (US), while providing key insights into the sources of stress and burnout in the field. The hope is that these insights can help facilitate systemic changes to support MS physicians to offer the quality care for their patients.

7. Newly Developed DAAE Score Shows Promise in Identifying Patient Risk for Transition to Progressive MS
Using a systematic literature review and advanced methods, a group of study investigators recently published findings on the DAAE score, a newly developed clinical tool for estimating individual patient risk to transition to secondary progressive multiple sclerosis (SMPS). Over a 5-year period, the easy-to-use tool estimated patient risk consistently across datasets internationally; however, it needs additional validation in larger cohorts to be used for clinical risk estimation and personalized care for individual people with MS.

8. Phase 3 ENSURE Program of Vidofludimus Calcium Continues Following Positive Futility Analysis
According to an announcement from Immunic, an Independent Data Monitoring Committee (IDMC) review of unblinded, interim data from the phase 3 ENSURE program assessing vidofludimus calcium in patients with relapsing multiple sclerosis (RMS) was positive, advising that the trials continue as planned. ENSURE, which includes 2 phase 3 trials (ENSURE-1; ENSURE-2), uses time to first relapse up to 72 weeks as the primary end point.

9. Simvastatin Fails to Reduce Disease Progression in Phase 3 MS-STAT2 Trial of Secondary Progressive Multiple Sclerosis
In the phase 3 MS-STAT2 trial (NCT03387670), treatment with simvastatin, a medication for high cholesterol, was safe and well tolerated, but demonstrated no evidence of benefit in reducing disability progression rates in patients with non-active progressing secondary progressive multiple sclerosis (SPMS). Investigators plan to release additional analyses from the trial that cover secondary outcomes, fluid biomarkers, and MRI.

10. Tolebrutinib Shows Positive Results in Slowing Disability Progression for Non-Relapsing Secondary Progressive MS in Phase 3 HERCULES Trial
Announced late-breaking results from the phase 3 HERCULES trial (NCT04411641) showed that tolebrutinib (Sanofi), a Bruton’s tyrosine kinase (BTK) inhibitor, had a significant effect on disability accumulation compared with placebo in patients with non-relapsing secondary progressive multiple sclerosis (nrSPMS). Presented at the 2024 European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) Congress , held September 18-20, in Copenhagen, Denmark, the findings from this trial are the first to reveal a significant slowing of disability progression in this patient population, for which there is a large unmet need

NeurologyLive® Year in Review 2024: Top Stories in Movement Disorders

Neurology Unwrapped: 2024’s Most Intriguing Conversations

NeurologyLive® Year in Review 2024: Top Stories in Stroke and Cerebrovascular Disease

Episode 130: The Promise Behind Cell Therapy Approaches in Epilepsy

NeurologyLive® Year in Review 2024: Most-Read FDA Approval Stories

NeurologyLive® Year in Review 2024: Top Conference Coverage Stories
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

What’s New in MS Research – November 2024
Reviewed by MSAA Chief Medical Officer Barry A. Hendin, MD
In this Article
The case for “starting strong”: two studies support early use of high-efficacy therapies, two large, long-term studies implicate infectious mononucleosis in ms risk, researchers find that menopause is not a culprit in ms disability progression, a delay of 31% in time to disability progression with tolebrutinib in non-relapsing spms, study links mediterranean diet adherence to better mental health and quality of life in ms, study finds high-dose vitamin d reduces ms activity after first event, updates to the mcdonald criteria for ms diagnosis, does steroid treatment for relapses improve the longer-term course of ms, better quality of life in ms tied to better quality of sleep, german study examines use of ms medications during pregnancy, can gait training be a step toward enhancing brain white matter in ms, investigational medication frexalimab shows enduring efficacy over 18 months, for more information.
The world’s leading experts in multiple sclerosis (MS) gathered in Copenhagen, Denmark in September 2024 to share the results of their latest research into the causes, course, and treatment of the disease. That gathering, the 40th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), showcased encouraging findings from a number of vital studies.These trials covered important findings on many topics, which include: investigational and approved disease-modifying therapies (DMTs); the roles of exercise, diet, and sleep in managing MS and enhancing quality of life; and approaches to diagnosing, monitoring, and predicting the course of MS. The brief summaries that follow give a sense of the breadth and depth of that research, and provide much hope for the future.
With two dozen therapies approved for the treatment of MS, clinicians have both a welcome opportunity and a considerable challenge when it comes to selecting the disease-modifying therapy (DMT) best suited for a particular individual with MS. One of the questions they face is whether to “start low and go slow” – initiating a lower- or moderate-efficacy therapy less likely to cause side effects and then moving to a high-efficacy therapy, as needed – or to “start strong” with a higher-efficacy therapy in hopes that the benefits it provides early in the course of MS will be worth the potential for more significant side effects.
While that decision has to be made in consultation between the physician and the patient, in the context of the individual’s specific situation, two recent studies drawing on data from the Swedish MS Registry provide support for early use of higher-efficacy DMTs.
The first study looked for an association between the type of therapy a person with MS first received and long-term disease progression. 1
Researchers compared the experiences of 2,390 people with relapsing forms of MS who were started on low-to-moderate efficacy therapies (LM-DMT) and 1,968 people with relapsing forms of MS who received first-line high-efficacy treatment (HE-DMT). There were 1,474 confirmed disability worsening events in the LM-DMT group compared with 982 such events in the HE-DMT cohort.
This translated into an unadjusted rate of 9.98 events per 100-person years in the lower/moderate-efficacy group versus 9.19 events in the high-efficacy group, which was a statistically significant difference. The HE-DMT group also had a lower rate of progression independent of relapse activity, although the difference between the two groups on this measure was not statistically significant.
The second study examined how the timing of initiating high-efficacy therapy in relapsing-remitting MS was associated with subsequent participation in the workforce. 2
Researchers analyzed data on 866 people with relapsing forms of MS aged 18 to 55 years. They paired those study participants, matching 433 who started a HE-DMT less than two years after disease onset with 433 who began HE-DMT therapy two-to-four years following onset of MS. The pairs were also matched by age at onset, sex, and relapse rate, as well as their Expanded Disability Status Scale (EDSS) score in the first two years after diagnosis. Seventy percent of study participants were female, and the participants’ average age of MS onset was 33.2 years. Study endpoints were receipt of a partial or full disability pension.
Over an average eight years of follow-up, the people who started a HE-DM two-to-four years after disease onset were almost two-times more likely to be receiving a disability pension than those who began a high-efficacy DMT within two years of developing MS.
The study’s authors concluded that earlier initiation of high-efficacy DMTs is associated with longer time in the workforce, adding that this finding “supports the benefit of early [high-efficacy therapy] shown in previous studies on disability outcomes.”
Researchers long have recognized a link between Epstein-Barr Virus (EBV) and multiple sclerosis, but the exact nature of that connection has been elusive. Now, two large studies analyzing decades’ worth of data have implicated infectious mononucleosis (IM), a contagious viral illness most often caused by EBV, as significantly increasing a person’s risk of developing MS.
In the first study, researchers drew on data from the Danish National Patient Register to identify 37,533 people who were diagnosed with IM caused by EBV. 3 The patients were diagnosed in the hospital setting and did not have a prior diagnosis of MS. Their average age at diagnosis of EBV-IM was 18.4 years; 47% were female. Researchers matched each of those patients with 10 people from the general population who were the same sex and age.
In analyzing data collected from January 1977 through December 2022, the researchers found that the people diagnosed with EBV-IM had an incidence rate of MS events of 43.1 per 100,000 person-years, almost three-times greater than that of the 15.0 per 100,000 person-years rate in their counterparts who had not been diagnosed with EBV-IM. Further, they found that this elevated risk persisted for up to 40 years after being diagnosed with infectious mononucleosis.
The degree of risk varied by the age at which a person was diagnosed with EBV-IM, the researchers added, citing a 3.34-fold increased risk in people found to have IM at ages 11 to 19 years versus a 2.46-fold elevation in risk for those diagnosed with IM at age 10 years or younger and a two-fold increase in risk for people who developed IM at age 20 years or older.
In the second study, researchers looked at data on 669,538 people diagnosed with IM in Ontario, Canada between April 1992 and March 2017. 4 From 1992 through March 2022, 2,475 of those people (0.4% of the total) were diagnosed with MS. Seventy-one percent of the people with MS were female. The median age at IM diagnosis was 18 years and the median age at MS onset was 31 years.
Delving more deeply into the data, the researchers found that the following characteristics were associated with an increased risk of developing MS following a diagnosis of infectious mononucleosis:
- Females, 1.9-times greater risk of developing MS relative to males
- IM diagnosis at age 10 to 25 years, 5.8-times greater risk of developing MS relative to diagnosis at 9 years or younger
- Previous need for mental health care, 1.2-times greater risk of developing MS relative to not receiving mental health care
Prior mental health care and older age at time of IM diagnosis also were associated with earlier onset of MS following infectious mononucleosis.
Menopause may bring unwelcome changes in terms of hot flashes, reduced bone density, and other issues, but progression of MS-related disability isn’t one of them, according to a report by Australian researchers. 5
Those investigators analyzed data on 1,117 women with MS who were receiving care at eight MS centers across Australia. Of the total, 656 women were pre-menopausal and 461 were post-menopausal. Researchers compared the two groups in terms of time to confirmed disability progression (CDP) and secondary-progressive multiple sclerosis (SPMS). While women in the postmenopausal group reached those milestones sooner than their pre-menopausal counterparts, statistical analysis showed that after adjusting for factors such as age at MS onset, disease duration, relapse history, and duration of use of high-efficacy disease-modifying therapies, menopause was no longer a predictor of CDP or SPMS in and of itself.
The researchers concluded, “The onset of menopause in women with MS is not the driving factor for disease progression, as measured using physical disability-based severity measures. These findings offer crucial insights and reassurance to women with MS navigating the menopausal transition.” They added, however, “Further research is needed to examine the impact of domain-specific markers of disability, including cognition.”
The oral disease-modifying therapy (DMT) tolebrutinib delayed time to onset of six-month confirmed disability progression by 31% compared to placebo in the Phase III HERCULES study involving more than 1,100 people with non-relapsing secondary-progressive multiple sclerosis (nrSPMS). 6,7
Tolebrutinib is an investigational medication that inhibits the activity of an enzyme called Bruton’s tyrosine kinase, or BTK. The enzyme helps direct the activity of different cells involved in neurological and immune function. In particular, it plays a role in the development and activation of B cells, immune cells that have been shown to contribute to the development of MS.
The 1,131 patients participating in HERCULES were an average age of 48.9 years and had an average time from MS symptom onset of 17.3 years. Sixty-two percent were female and 77% had received at least one prior DMT. Study participants were randomized 2:1 to receive 600 mg of tolebrutinib daily or placebo.
Sanofi, the company developing the BTK inhibitor, reported that 10% of study participants receiving tolebrutinib experienced confirmed disability improvements, compared to 5% of people receiving placebo.
The company also reported that liver enzyme elevations three-times greater than the upper limit of normal (ULN) occurred in 4.1% of people receiving tolebrutinib compared to 1.6% of those in the placebo group. One half of one percent of patients in the tolebrutinib group had increases more than 20-times the ULN in blood levels of the liver enzyme alanine aminotransferase (ALT). Those elevations all occurred in the first 90 days of treatment, the company said.
A Sanofi press release noted that all but one case of liver enzyme elevations resolved without further medical intervention. The press release also explained that, “Prior to the implementation of the revised study protocol with more stringent monitoring, one participant in the tolebrutinib arm received a liver transplant and died due to post-operative complications.”
To date, the implementation of more frequent monitoring has mitigated such serious effects on the liver. Other deaths in the trial were assessed as unrelated to treatment according to investigators; deaths were equal across the placebo and tolebrutinib arms at 0.3%. Liver enzyme elevations have been seen in a number of BTK inhibitors being developed by various companies.
There currently are no therapies approved by the Food and Drug Administration (FDA) specifically for the treatment of nrSPMS.
Results from the Phase III GEMINI 1 and GEMINI 2 studies evaluating tolebrutinib in relapsing MS also were announced at the ECTRIMS meetings. Those studies compared the BTK inhibitor to Aubagio ® (teriflunomide), an oral DMT approved by the FDA for the treatment of relapsing forms of MS. 7,8
The two studies enrolled more than 1,800 people (974 in GEMINI 1 and 899 in GEMINI 2) from 42 different countries. Study participants’ average age was 36.5 years, and their average time from diagnosis was 4.3 years. Two-thirds of the study participants were female and 63% had not received prior treatment with a DMT. Their average Expanded Disability Status Scale (EDSS) score was 2.38, indicating minimal or mild disability, and study participants had experienced an average of 1.2 relapses in the year prior to entering the study.
The primary endpoint for both GEMINI 1 and GEMINI 2 was a statistically significant improvement in annualized relapse rate (ARR) for tolebrutinib compared to Aubagio. Unfortunately, neither study met that primary endpoint, with the ARR for tolebrutinib actually slightly higher in GEMINI 1 and identical to that of Aubagio in GEMINI 2.
However, in a pooled analysis of data from the two studies, tolebrutinib delayed the time to onset of six-month confirmed disability worsening by 29%, in line with the 31% delay in confirmed disability progression seen in people with nrSPMS in the HERCULES study.
Preliminary analysis of safety data from GEMINI 1 and 2 showed that adverse events seen in the tolebrutinib and Aubagio study arms were generally comparable. Liver enzyme elevations more than three-times the ULN were recorded in 5.6% of people receiving tolebrutinib compared with 6.3% of participants receiving Aubagio. Those liver enzyme elevations resolved without further medical intervention, Sanofi reported. Deaths were balanced across the Aubagio and tolebrutinib arms, at 0.2% and 0.1% respectively, and were assessed as unrelated to treatment by investigators.
A fourth Phase III study of tolebrutinib, the PERSEUS trial evaluating the BTK inhibitor in primary-progressive MS, is ongoing, with results expected in the second half of 2025.
A study involving 489 people with MS found that the more participants followed a Mediterranean Diet, the more likely they were to have better mental health and quality of life (QoL). 9
The study, which drew on data from the United Kingdom Multiple Sclerosis Register (UKMSR), assessed participants’ dietary intake in 2016 and again in 2022. Based on self-reported eating habits, each study participant was assigned a score from 0 to 9 on the alternate Mediterranean Diet (aMED) instrument. Higher scores signify higher adherence to the diet, which emphasizes eating fruits, vegetables, whole grains, olive oil, and lean meats, while limiting or avoiding red meat and sugar, and consuming wine in moderation, if at all. The diet has been shown to have anti-inflammatory properties and is favored by many cardiologists and other physicians for the cardiovascular benefits it has demonstrated.
The researchers also measured participants’ degree of anxiety and depression using the Hospital Anxiety and Depression Scale, mental QoL with the Multiple Sclerosis Impact Scale, and overall quality of life with the EuroQol 5 Dimension. They also adjusted results to take into account factors including 2016 health outcomes and 2022 total energy intake (food and beverages consumed), age, sex, type of MS, and medication use.
They found that higher aMED scores in 2016 predicted reduced anxiety and a reduced risk for moderate or severe depression. Similarly, people with the highest degree of adherence to a Mediterranean diet (those with aMED scores of 7-9) had a 34% reduction in risk for low mental quality of life relative to those with the lowest degree of following a Mediterranean diet (participants with aMED scores of 0-3).
Researchers in the United States and other countries already are examining whether the Mediterranean diet can slow the progression of physical disability in MS, and the results they have achieved thus far are encouraging, though not definitive. Given these promising results so far, as well as the need for people with MS to protect their cardiovascular health, this study provides another reason for people with MS to talk with their clinicians about the potential benefits of “going Mediterranean.”
Taking high doses of Vitamin D3 every two weeks reduces multiple sclerosis disease activity in people who have had an initial demyelinating event, according to a team of French investigators. 10
The researchers recruited 314 people ages 18 to 55 years who had experienced a clinically isolated syndrome – such as optic neuritis or some other episode potentially indicating MS – in the past 90 days. All study participants had low serum levels of Vitamin D, which is a risk factor for multiple sclerosis, and magnetic resonance imaging (MRI) findings that met the Swanton criteria for diagnosing MS.
Half of the study participants were assigned to receive 100,000 International units (IU) of oral cholecalciferol, also known as Vitamin D3, every two weeks for 24 months or until they had evidence of disease activity (EDA). The other half received placebo. Assignment to the two groups was made in random fashion, and researchers did not know which people were receiving cholecalciferol and which were taking placebo. EDA was defined as a relapse or the presence of new T2 lesions on MRI performed at three, 12, and 24 months. The study analysis conducted after two years included 303 of the 314 people who initially enrolled.
Over the course of the study, 74.1% of those receiving placebo had evidence of disease activity, as compared to 60.3% of people receiving cholecalciferol. That reduction in risk in the Vitamin D group was statistically significant, and was seen regardless of patient age, gender, and number of lesions on baseline MRI. Further, the median time to EDA was 224 days in the placebo group versus 432 days in the Vitamin D group, with that difference again being statistically significant. The study’s authors reported that the dose of 100,000 IU of cholecalciferol, which is considered a high dose of the vitamin, every two weeks was well tolerated.
Those researchers concluded, “Together with the good safety profile, these data support high dose [Vitamin D] supplementation in early MS.”
Vitamin D supplementation in people who have MS or who are at an elevated risk for developing the disease seems logical. After all, if Vitamin D deficiency increases the chances of developing multiple sclerosis, shouldn’t helping people achieve normal or higher-than-normal levels of the nutrient reduce their risk of MS and improve outcomes in people already diagnosed with the disease?
Unfortunately, that simple, understandable question has yielded a complex, difficult-to-interpret set of answers. The many studies exploring Vitamin D supplementation in MS have yielded an array of findings. Some – like this study – have provided encouraging results. Others have found no or little benefit from the use of Vitamin D. The matter is complicated by the fact that the studies have used different research methods, included different populations, assessed different types and doses of Vitamin D, and have focused on different outcomes.
If you are interested in taking Vitamin D supplements, or are already taking them, it is important to talk with your clinician to determine what the available evidence indicates is the best approach for you and your situation.
Editor’s note: Individuals looking to take Vitamin D supplements as part of their treatment regimen should consult their physician. Continued high-dose Vitamin D supplementation can lead to life-threatening conditions, including kidney failure and heart arrythmias.
The tools and techniques available to clinicians for identifying MS have improved dramatically in the 23 years since Professor Ian McDonald and colleagues proposed the diagnostic criteria that bear his name. To make the most of those advances, an international team of experts has proposed comprehensive updates to the criteria.
In one of the most-anticipated sessions at the ECTRIMS meeting, Xavier Montalban, MD, PhD, outlined the revisions recommended by that group, the International Advisory Committee on Clinical Trials in Multiple Sclerosis. 11
Dr. Montalban, who practices at Vall d’Hebron University Hospital in Barcelona, Spain and is a leading MS researcher, explained that the updates are intended to speed and simplify the recognition of multiple sclerosis while enhancing diagnostic accuracy.
One of the main recommended changes focuses on the long-standing requirement that evidence of disease be both disseminated in space (DIS), meaning imaging showing MS lesions in various areas of the central nervous system (CNS), and disseminated in time (DIT), meaning apparent demyelinating events occurring on two or more occasions rather than just once. The proposed updates would drop the need to demonstrate DIT.
Other changes include:
- Allowing radiologically isolated syndrome (RIS) – the incidental discovery on magnetic resonance imaging (MRI) of CNS lesions characteristic of MS in people with no MS symptoms – to be diagnosed as MS in specific situations
- Including the optic nerve as one of the CNS areas, or “topographies,” that can be counted toward dissemination in space
- Applying stricter criteria for diagnosing people age 50 years or older who have headache or vascular disorders
- Employing a single, unified framework for diagnosing relapsing-remitting MS and primary-progressive MS, with the latter requiring evidence of clinical progression over at least 12 months
- Assessing levels of kappa free light chains (kFLCs), proteins that can be produced when there is chronic inflammation in the intrathecal space between the spinal cord and its protective membranes, to aid in diagnosis
- Using the myelin oligodendrocyte glycoprotein IgG Antibody (MOG-IgG Ab) blood test to confirm the diagnosis of MS in children and adolescents
- Specifying that the central vein sign and paramagnetic rim lesions – two indicators of MS identified on MRI – are optional tools for aiding in diagnosis in certain circumstances
Dr. Montalban, who chaired the committee proposing these updates, said that next steps are developing a paper outlining the revised criteria and an accompanying diagnostic algorithm, consulting with the wider MS community, and launching a global education campaign.
When clinicians prescribe corticosteroids to people experiencing MS relapses, the goal is to speed recovery from that acute event, not to slow disability worsening over time.
Now, however, a study involving 3,673 people with MS who received steroids to treat a relapse suggests that the medication may have a favorable longer-term impact. 12 Study participants had clinically definite MS and an Expanded Disability Status Scale (EDSS) score of 3 or greater (indicating at least moderate disability in one functional system or mild disability in three or four functional systems). The study’s primary outcomes measure was disability worsening confirmed over 12 months, as measured by change in EDSS score.
The study participants had a total of 5,809 relapses, 4,671 of which were treated with corticosteroids, and 1,138 of which were untreated. Over a median 5.2 years of follow-up, 32.7% of people in the overall study group had confirmed disability worsening. People who had not received a corticosteroid for their relapse were roughly 1.5-times more likely to experience disability worsening than those who had been treated with steroids.
The study’s authors concluded, “Corticosteroid treatment of MS relapses may impact not only recovery speed, but also the severity of residual structural damage.”
Call it a victory for truth in advertising. A Danish study involving 405 people with MS confirms what all those mattress company spokespeople tell us: a good night’s sleep really does make a huge difference in quality of life. 13
Study participants were ages 18-to-65 years old and had Expanded Disability Status Scale (EDSS) scores of 7.5 or less, indicating mild to moderate degrees of MS-related impairment. Based on their scores on the Pittsburgh Sleep Quality Index (PSQI) instrument, researchers categorized them as “good sleepers,” “poor sleepers,” and “very poor sleepers.”
The researchers then looked for differences between the three sleep groups in terms of scores on the MS-specific health-related quality of life (HRQoL) instrument and the Functional Assessment of MS (FAMS) questionnaire, both of which are scientifically validated measures.
Not surprisingly, better sleep quality was associated with better health-related quality of life. Similarly, average scores on the FAMS instrument were highest among good sleepers and lowest among very poor sleepers.
While the results may have been predictable, they nonetheless serve a valuable purpose in confirming and underscoring the importance of good sleep for people with multiple sclerosis. Of course, getting a good night’s sleep is easier said than done when dealing with the various symptoms of MS. Hopefully, this study will provide people with MS and their clinicians with reason to revisit problem-solving strategies for the spasticity, urinary urgency/frequency, or other manifestations of MS that prevent people from sleeping soundly.
Taking disease-modifying therapies (DMTs) during pregnancy did not increase risk for miscarriage, preterm birth, or delivering a child with major birth defects, according to a German study examining 3,722 pregnancies in women with MS. 14
The study reached that welcomed conclusion by comparing the outcomes of 2,885 pregnancies marked by maternal exposure to DMTs with the outcomes of 837 pregnancies without DMT exposure.
However, women with MS were almost twice as likely as women without MS to have a baby who was small for gestational age (SGA). The study found that among all pregnancies in women with MS – including those that were and were not marked by exposure to DMTs – 18.8% of deliveries involved a baby who was SGA, while that rate in the general German population of pregnant women is 10%.
The researchers also looked at how the type of DMT used affected pregnancy outcomes. They found that pregnancies marked by exposure to sphingosine-1-phosphate-receptor-modulators (S1P group) and anti-CD-20-antibodies (CD-20 group) – two classes of higher-efficacy DMTs – were about 1.5-times more likely to result in delivery of a baby who was small for gestational age relative to a pregnancy in which a woman with MS did not take DMTs.
They also reported that use of Tysabri ® (natalizumab) later in pregnancy and exposure to S1P group medications were associated with reduced birth weight. Further, the investigators found that the risk of severe infections during pregnancy was highest among women taking S1P agents and fumarate-class DMTs.
We all know that the brain controls movement, but can movement have an impact on brain anatomy in people with MS? A small study from Germany raises that intriguing question. 15
The study involved 31 people with MS who completed four weeks of gait training (physical therapy to improve walking). The study participants had a median age of 44 years and a median Expanded Disability Status Scale (EDSS) score of 2.5, indicating a mild degree of disability. Researchers used magnetic resonance imaging (MRI) to evaluate the participants’ brains at baseline and at the end of the study. The imaging included creation of “fractional anisotropy (FA) maps,” which assess white-matter integrity. White matter is a network of nerve fibers that plays a key role in balance, walking, learning, and problem-solving, among other functions.
After the four-week study period, 11 of the 31 participants had increased the distance they could walk in two minutes by 5% or more from their baseline performance. Researchers termed these people responders. People who did not increase their baseline distance by at least 5% were categorized as non-responders.
The findings from this study were quite interesting. On baseline MRI, there were no noteworthy differences in the FA maps of the people who turned out to be responders and those who were non-responders. However, at the end of the training, the responders showed evidence of enhanced white matter integrity in areas involved in motor function.
It is important to note that this was a small study. Its findings will need to be replicated in a larger trial, and the practical implications of those findings will need to be determined. However, the prospect of people with MS being able to enhance the integrity of brain tissue (along with the functioning of brain tissue) through supervised exercise, remains exciting.
The investigational disease-modifying therapy (DMT) frexalimab demonstrated a sustained reduction in MS disease activity and a continued favorable safety profile over an 18-month period, researchers reported. 16
Frexalimab is a monoclonal antibody, or a protein that affects immune response. It blocks the CD40/CD40L pathway, which activates immune system T cells and B cells and has been implicated in the development of MS.
In a Phase II trial of the medication, 125 people with relapsing MS were assigned to receive either 1,200 mg of frexalimab administered intravenously (IV) every four weeks, 300 mg of frexalimab administered by subcutaneous (SC) injection every two weeks, or corresponding doses of placebo. At the end of the 12-week trial, the 1,200-mg dose of IV frexalimab decreased new gadolinium-enhancing (Gd+) T1 lesions by 89% compared to placebo.
People completing the trial were invited to enter its open-label extension (OLE). During that period of ongoing monitoring, study participants who had been randomly assigned to placebo in the main study switched over to receive frexalimab. Further, the dose of subcutaneous frexalimab was increased to 1,800 mg every four weeks, providing medication exposure comparable to that of 1,200 mg given intravenously every four weeks.
Researchers reported that the treatment benefit seen in the 12-week study was maintained over 48 weeks in the OLE period.
At the ECTRIMS meeting in September, those researchers provided follow-up data extending out to 18 months for 111 study participants. They reported that the number of Gd+ T1 lesions remained low both in participants who continued frexalimab and in those who had switched from placebo to frexalimab, with the average number of T1 lesions being:
- 0.1 in those who received intravenous (IV) frexalimab from the outset
- 0.4 in those who received subcutaneous (SC) injection frexalimab from the outset
- 0.0 in those who switched from placebo to IV frexalimab at Week 12
- 0.2 in those who switched from placebo to SC frexalimab at Week 12
The researchers added that no new safety signals were seen over 18 months of frexalimab treatment, with the most common reported adverse events being colds/sore throat (experienced by 13% of study participants), COVID-19 (12%), and headache (11%).
- Spelman T, Glaser A, Hilert J. Immediate high-efficacy treatment in multiple sclerosis is associated with long-term reduction in progression independent of relapse activity (PIRA) compared to low-moderate efficacy treatment – a Swedish MS Registry study. ECTRIMS 2024. P842/178. Mult Scler J . 2024; 30:(3S):651.
- He A, Sebside F, McKay K, et al. Earlier high-efficacy therapy is associated with less work disability in relapsing multiple sclerosis: a nationwide observational cohort study. Abstract P341/1258. ECTRIMS 2024. Mult Scler J . 2024; 30:(3S).
- Kopp T, Magyari M, Winther Torring C, et al. A Danish observational study on the risk of multiple sclerosis associated with infectious mononucleosis caused by Epstein-Barr virus over 40 years. Abstract P075/572. ECTRIMS 2024. Mult Scler J . 2024;30:(3S):181.
- Rotstein D, Marrie RA, Tremlett H, et al. Risk and onset of multiple sclerosis after infectious mononucleosis (IM): a population-based study of >650,000 cases of IM. ECTRIMS 2024. P078/385. Mult Scler J . 2024;30:(3S):184.
- Bridge F, Sanfilippo P, Skibina O, et al. Driving forces: investigating menopause’s influence on MS disability progression. Abstract P091/464. ECTRIMS 2024.
- Fox RJ, Bar-Or A, Traboulsee A, et al. Efficacy and safety of tolebrutinib versus placebo in non-relapsing secondary progressive multiple sclerosis: results from the Phase 3 HERCULES trial. Abstract O136/4027. ECTRIMS 2024.
- Sanofi. Tolebrutinib demonstrated a 31% delay in time to onset of confirmed disability progression in non-relapsing secondary progressive multiple sclerosis phase 3 study. Press release. September 24, 2024. Paris, France. Accessible at https://www.sanofi.com/en/media-room/press-releases/2024/2024-09-20-09-30-00-2949552 . Accessed November 5, 2024.
- Oh J, Arnold DL, Cree BAC, et al. Efficacy and safety of tolebrutinib versus teriflunomide in relapsing multiple sclerosis: results from the Phase 3 GEMINI 1 and 2 trials. ECTRIMS 2024. O135/4026. Mult Scler J . 2024;30:(3S):1145.
- Yu M, Reece J, Coe S, et al. A higher Mediterranean Diet score is prospectively associated with better mental health and quality of life in a national multiple sclerosis cohort. ECTRIMS 2024. P370/524. Mult Scler J . 2024; 30:(3S):390.
- Thouvenot E, Laplaud D, Lebrun-Frenay, et al. High-dose cholecalciferol reduces multiple sclerosis disease activity after a clinically isolated syndrome: results of a 24-month placebo-controlled randomized trial (D-lay MS). Abstract O065/1291.ECTRIMS 2024.
- Montalban X. 2024 revisions of the McDonald criteria. ECTRIMS 2024.
- Roberts J, Sharmin S, Horakova D, et al. Corticosteroid treatment of multiple sclerosis relapses is associated with lower disability worsening over 5 years. ECTRIMS 2024. P333/214. Mult Scler J . 2024;30:(3S):357.
- Langeskov-Christensen M, Boesen F, Trenel P, et al. Poor sleep has a deleterious impact on health-related quality of life in people with multiple sclerosis – The Danish MS hospitals rehabilitation study. ECTRIMS 2024. P114/1662. Mult Scler J . 2024;30:(3S):210.
- Bast N, Dost-Kovalsky K, Haben S, et al. Impact of disease-modifying therapies on pregnancy outcomes in multiple sclerosis: a cohort study from the German Multiple Sclerosis and Pregnancy Registry. Abstract O090/320. ECTRIMS 2024.
- Helmlinger B, Seebacher B, Opriessnig P. Changes in white matter integrity after gait training in people with multiple sclerosis. ECTRIMS 2024. P181/893. Mult Scler J . 2024; 30:(3S):253.
- Giovannoni G, Granziera C, Mao-Draayer Y, et al. Safety and efficacy of frexalimab in the treatment of relapsing multiple sclerosis: 18-month results from the Phase 2 open-label extension. Abstract O066/242. ECTRIMS 2024.
For general information or to speak with a trained Client Services Specialist, please call MSAA’s Helpline at (800) 532-7667, extension 154 . Questions to MSAA’s Client Services department may also be emailed to [email protected] .
Written by Tom Garry, Medical Writer Reviewed by Dr. Barry Hendin , MSAA Chief Medical Officer Edited by Susan Wells Courtney, MSAA Senior Writer
About Hoff Communications
Multiple Sclerosis Research News
Top headlines, latest headlines.
- A New Era of Treating Neurological Diseases
- Treating MS: Meds, CBT
- Promising Combined Therapy for MS
- Molecular Mechanism Behind MS
- MS May Protect Against Alzheimer's
- New Way Inflammation Impacts Cell Communication
- New T Cells, Genes Related to Immune Disorders
- MS: Epstein-Barr Virus, Brain Cross-Reactivity
- Selective MS Treatment Strategy
- Flicker Stimulation Shines for Epilepsy
Earlier Headlines
Thursday, july 25, 2024.
- Can a Computer Tell Patients How Their Multiple Sclerosis Will Progress?
Wednesday, April 24, 2024
- For Immigrants to Canada, Risk of Multiple Sclerosis Increases With Proportion of Life Spent There, Study Finds
Tuesday, March 26, 2024
- Common Household Chemicals Pose New Threat to Brain Health, Study Finds
Thursday, March 21, 2024
- Research Offers Hope for Preventing Post-COVID 'brain Fog' By Targeting Brain's Blood Vessels
Wednesday, March 13, 2024
- Recreational Activities Such as Golfing, Gardening May Be Associated With Increased ALS Risk Among Men
Tuesday, February 20, 2024
- Fixing Rogue Brain Cells May Hold Key to Preventing Neurodegeneration
Thursday, February 8, 2024
- Analysis of Biological Networks Helps Explain the Complexity of Multiple Sclerosis
Tuesday, February 6, 2024
- Discovery May Enable an Effective Long-Term Lupus Treatment
Tuesday, January 9, 2024
- Severe MS Predicted Using Machine Learning
Monday, December 11, 2023
- Study Reveals a Protein Called Snail May Play a Role in Healing Brain Injury
Friday, December 8, 2023
- Potential New Drug Treatment for Multiple Sclerosis
Monday, November 6, 2023
- A Blood Test Shows MS Worsening 1 to 2 Years Before It Happens
Monday, September 25, 2023
- Depression, Anxiety May Be Among Early Signs of MS
- Marker for Brain Inflammation Finally Decoded
Tuesday, August 8, 2023
- Possible Biomarker of MS-Like Autoimmune Disease Discovered
Wednesday, July 19, 2023
- Treatment at the First Signs of MS Could Mean Lower Risk of Disability Later
Wednesday, July 12, 2023
- Oral Medication Is the Leading Choice for Multiple Sclerosis Treatment
Thursday, July 6, 2023
- Multiple Sclerosis: New Biomarker Confirmed for Early Diagnosis
Wednesday, June 28, 2023
- Genetic Variant Linked With Faster Progression of Multiple Sclerosis
Thursday, June 15, 2023
- Pregnancy Hormone Repairs Myelin Damage in MS Mouse Model
Monday, June 12, 2023
- Can This Medication Reverse MS? Brain Biomarker Shows It Can
Thursday, March 16, 2023
- Children at Risk of Multiple Sclerosis Often Go Undetected in Early Stages
Wednesday, March 8, 2023
- First Nasal Monoclonal Antibody Treatment for COVID-19 Shows Promise for Treating Virus, Other Diseases
Monday, March 6, 2023
- Potential Treatment of Autoimmune Diseases Revealed in New Study
Wednesday, February 1, 2023
- Researcher Takes Another Step Toward Discovering How a Brain Molecule Could Halt MS
Wednesday, December 21, 2022
- Stem Cell Transplants May Delay Disability Longer Than Some MS Medications
Monday, December 12, 2022
- Light Therapy Relieves Fatigue Syndrome in Multiple Sclerosis
Friday, November 18, 2022
- New Study Identifies Connection Between Diabetes Medications, Multiple Sclerosis
Thursday, November 10, 2022
- Immune System Reboot in MS Patients
Friday, November 4, 2022
- Researchers Offer Roadmap for Identifying New Neuroprotective Treatments by Leveraging Sex Differences
Thursday, October 6, 2022
- Tofacitinib Shows Promise in Scleroderma Patients, Researchers Optimistic for Next Phase of Study
- Study Advances Knowledge of Role of Brain Pathology and Cognitive Fatigue in Multiple Sclerosis
Tuesday, September 20, 2022
- Fish to Help in Search for MS Drugs
Thursday, September 1, 2022
- Simple Blood Test Predicts Neurotoxic Complications of CAR-T Cell Therapy
Wednesday, August 24, 2022
- Health Care Spending May Help Explain Link Between Multiple Sclerosis and Latitude
Wednesday, August 10, 2022
- Quality of Life With Multiple Sclerosis May Depend on Several Factors
- Multiple Sclerosis Drug Works in a Surprising Way
Wednesday, July 6, 2022
- Virtual Reality Technology Could Strengthen Effects of Traditional Rehabilitation for Multiple Sclerosis
Thursday, May 26, 2022
- Harnessing the Immune System to Treat Traumatic Brain Injury in Mice
Friday, May 20, 2022
- Uncovering New Details of the Brain's First Line of Defense
Wednesday, May 11, 2022
- Multiple Sclerosis: Glatiramer Acetate Compatible With Breastfeeding, Study Suggests
Wednesday, April 27, 2022
- Findings Open Way for Personalized MS Treatment
Tuesday, April 26, 2022
- A New Treatment Reduces Inflammation in Multiple Sclerosis Mice Models
Thursday, April 14, 2022
- Could Releasing ‘handbrake’ Immune Cells Help Supercharge Immunity?
Wednesday, April 13, 2022
- Total Economic Burden of MS in United States Is More Than $85 Billion
Tuesday, April 12, 2022
- Behavioral Treatment for Deficits of Facial Affect Recognition in Multiple Sclerosis
Thursday, March 24, 2022
- Researchers Develop New Antibody Test to Diagnose Multiple Sclerosis
Tuesday, March 22, 2022
- Research Suggests Interrupting Immune Response Improves Multiple Sclerosis Outcomes
Friday, March 4, 2022
- New Discovery May Help Reduce Side Effects of Multiple Sclerosis Drugs
Tuesday, March 1, 2022
- Milk May Exacerbate MS Symptoms
Friday, February 18, 2022
- Myelination Determines the Nerve Cell Power of Inhibition, Study Finds
Wednesday, February 16, 2022
- Multiple Sclerosis: Study With Twins Untangles Environmental and Genetic Influences
Friday, January 28, 2022
- New Genetic Clues on Multiple Sclerosis Risk
Thursday, January 27, 2022
- Meat, MS and the Microbiome
Tuesday, January 18, 2022
- New MRI Technique Could Improve Diagnosis and Treatment of Multiple Sclerosis
Thursday, January 13, 2022
- Epstein-Barr Virus May Be Leading Cause of Multiple Sclerosis
Monday, January 10, 2022
- Personalizing Treatment for Severe Limb Injuries
Thursday, January 6, 2022
- Can a Human Microglial Atlas Guide Brain Disorder Research?
- LATEST NEWS
- Health & Medicine
- Diseases & Conditions
- Alzheimer's Research
- Amyotrophic Lateral Sclerosis
- Attention Deficit Disorder
- Back and Neck Pain
- Birth Defects
- Bladder Disorders
- Blood Clots
- COVID and SARS
- Cervical Cancer
- Bladder Cancer
- Multiple Myeloma
- Pancreatic Cancer
- Brain Tumor
- Colon Cancer
- Breast Cancer
- Ovarian Cancer
- Lung Cancer
- Mesothelioma
- Skin Cancer
- Prostate Cancer
- Cerebral Palsy
- Chikungunya
- Chronic Fatigue Syndrome
- Cold and Flu
- Crohn's Disease
- Cystic Fibrosis
- Dengue Fever
- Down Syndrome
- Eating Disorder Research
- Encephalitis
- Epilepsy Research
- Erectile Dysfunction
- Fibromyalgia
- Gastrointestinal Problems
- HIV and AIDS
- Headache Research
- Hearing Loss
- Heart Health
- Cholesterol
- Stroke Prevention
- Heart Disease
- Hormone Disorders
- Hypertension
- Infectious Diseases
- Insomnia Research
- Irritable Bowel Syndrome
- Kidney Disease
- Liver Disease
- Lung Disease
- Lyme Disease
- Mental Health Research
- Multiple Sclerosis Research
- Mumps, Measles, Rubella
- Muscular Dystrophy
- Osteoporosis
- Parkinson's Research
- Prostate Health
- Restless Leg Syndrome
- Sickle Cell Anemia
- Sleep Disorder Research
- Thyroid Disease
- Triglycerides
- Tuberculosis
- Medical Topics
- Accident and Trauma
- Alternative Medicine
- Birth Control
- Bone and Spine
- Chronic Illness
- Controlled Substances
- Dietary Supplements and Minerals
- Epigenetics
- Food Additives
- Foodborne Illness
- Foot Health
- Gene Therapy
- Health Policy
- Human Biology
- Immune System
- Joint Health
- Medical Imaging
- Nervous System
- Pain Control
- Personalized Medicine
- Pharmacology
- Psychology Research
- Wounds and Healing
- PHYSICAL/TECH
- ENVIRONMENT
- SOCIETY & EDUCATION
- Infectious Disease Outbreaks and Auto-Detection
- Diet and Brain Health
- New 'All-Optical' Nanoscale Sensors of Force
- Waves of Early Human Migrations: Ancient DNA
- Origins of a Fast Radio Burst
- Semiconductors: Maskless Photolithography
- Surges in Estrogen and Binge Drinking
- Early Use of Substances and The Brain
- Parma Wallaby Needs Fox-Free Safe Havens
- Virus That Threatened Humanity Opens the Future
Trending Topics
Strange & offbeat.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 28 December 2024
New autoimmune disorder development after immune reconstitution therapy for multiple sclerosis
- Nataša Giedraitienė 1 ,
- Rasa Kizlaitienė 1 &
- Gintaras Kaubrys 1
Scientific Reports volume 14 , Article number: 30991 ( 2024 ) Cite this article
791 Accesses
2 Altmetric
Metrics details
- Multiple sclerosis
Immune reconstitution therapy (IRT) is a relatively new and highly effective treatment option for multiple sclerosis (MS). Uncertainty regarding the development of autoimmune disorders (ADs) after some therapies remains. The aim of this study was to assess new AD development after IRT in MS patients and to describe the nature of those ADs and the time to onset. A total of 179 patients with relapsing multiple sclerosis (37 after autologous haematopoietic stem cell transplantation (AHSCT), 19 after alemtuzumab (ALE) and 123 after cladribine (CLA) treatment) over a ten year period were included in the study. ADs were observed in 6 patients (16.2%) after AHSCT, 8 patients (42.1%) after ALE and 2 patients (1.6%) after CLA treatment. ADs developed earlier after ALE infusions, but later after AHSCT except for cytopenias. Neurologists should be attentive to the development of secondary ADs after ALE and AHSCT in MS patients.
Similar content being viewed by others
New autoimmune diseases after autologous hematopoietic stem cell transplantation for multiple sclerosis

B cells in multiple sclerosis — from targeted depletion to immune reconstitution therapies

Efficacy and safety of rituximab in multiple sclerosis and neuromyelitis optica spectrum disorder
Introduction.
Immune reconstitution therapy (IRT) is a form of high-efficacy treatment for relapsing multiple sclerosis (RMS) that has the potential to induce long-term or even permanent drug-free remission in people with multiple sclerosis (MS) 1 , 2 . These therapies deplete components of the immune system with the aim of allowing the immune system to renew itself. Cladribine (CLA), which is orally administered in two yearly treatment courses, and the monoclonal antibody alemtuzumab (ALE), which is administered by intravenous infusions for two yearly treatment courses are frequently categorized as IRT 3 , 4 . Autologous haematopoietic stem cell transplantation (AHSCT), which has been commonly used for a long time for the treatment of haematological cancers, is also increasingly used for the treatment of patients severely affected by autoimmune diseases, including MS 5 . AHSCT could be considered the strongest IRT for MS 2 , 6 . AHSCT induces ablation of the immune system via the removal of inflammatory and autoreactive cells and results in immunosuppression that is relatively short and depends on the intensity of the conditioning regimen 7 , 8 , 9 . Some drugs may be difficult to place according to the MS disease-modifying therapy (DMT) classification; for example, anti-CD20 depleting therapies might also have some characteristics of IRT, as they modify the immune cell profile upon reconstitution 1 , 7 .
IRT is a new treatment option for MS, which is an autoimmune disease; however, some data have shown that IRT can potentially induce secondary autoimmune diseases (ADs) 10 , 11 , 12 , 13 . Secondary ADs were first described after haematopoietic stem cell transplantation (HSCT), mostly after allogenic procedures, which were undertaken for haematologic diseases 12 , 13 . Thyroid disorders are the most common endocrine disorders occurring after transplantation 14 , 15 . However, the incidence rate of thyroid dysfunction after HSCT was calculated in the population with different disorders treated with HSCT and different conditioning regimens, even in those patients in whom ALE was used for the conditioning regimen 16 . Some of the other most common autoimmune diseases after HSCT include autoimmune cytopenias, which can occur in up to 5–6% of patients. Among them, autoimmune thrombocytopenia is the most common and occurs in 2% of patients treated with AHSCT 12 , 13 , 16 , 17 , 18 , 19 , 20 .
ALE is considered a highly effective disease-modifying therapy for RMS. However, its utility is limited because it increases the risk of infections and, in particular, secondary ADs. The current perspective suggests that ALE profoundly alters the circulating lymphocyte phenotype and describes a reconstituted immune system characterized by T-cell activation, increased regulatory control of IL-17 producing effector T cells and CD20 + T cells, and reduced control of B cells, which increase the risk of autoimmune events 21 , 22 . On the other hand, studies have shown that autoimmunity arises in patients with greater T-cell apoptosis and cell cycling in response to alemtuzumab-induced lymphocyte depletion, a phenomenon that is driven by higher levels of IL-21 23 , 24 . Secondary thyroid ADs are the most common type of AD that occurs after treatment with ALE. Thyroid disorders have been reported in approximately one-third of patients with no previous history of thyroid dysfunction, with most studies reporting a prevalence between 30 and 45% 25 , 26 , 27 , 28 , 29 . Thyroid AD mostly develops within 5 years after ALE infusion, with a peak incidence in the third year from the first course 27 , 30 . Other secondary ADs, such as immune thrombocytopenic purpura (1–3%) 31 , 32 , glomerulonephritis 33 , 34 and haemolytic anaemia 35 , 36 , have also been rarely reported after ALE infusions.
CLA safety data have shown that CLA is not associated with immune-mediated diseases 37 . In contrast to ALE and AHSCT, ADs have not yet been reported after CLA tablets, except for one case of glomerulonephritis, which occurred shortly after the fourth CLA treatment course 38 . IRT is a relatively novel therapy for MS, and it is currently unclear with some therapies whether the development of new ADs is therapy specific, or time-limited or whether specific ADs develop at predictable intervals after therapy exposure. In our study, we provided the follow-up data of patients with MS treated with IRT, with the aim of determining the proportion of patents who develop new ADs and to describe the nature of those ADs and the time to onset.
A total of 179 patients with MS treated with IRT were included in the study. All patients had RMS. Thirty-seven patients (20.7%) were treated with AHSCT, 19 patients (10.6%) were treated with ALE infusions, and 123 patients (68.7%) were treated with CLA tablets. Patient disease characteristics and details for the patients by number of ALE or CLA courses are shown in Table 1 . The mean duration of follow-up in patients treated with AHSCT was 53.6 ± 27.5 months, that in patients treated with ALE was 61.1 ± 20.1 months, and that in patients treated with CLA was 31.6 ± 13.3 months.
New autoimmune diseases were diagnosed in 16 patients (8.9%) during follow-up, with 11 (6.1%) females and 5 (2.8%) males affected: in 6 patients (16.2%) after AHSCT, in 8 patients (42.1%) after ALE and in 2 patients (1.6%) after CLA treatment (Table 2 ). The majority of ADs after IRT were of thyroid origin (n = 13, 81.3%), of which hyperthyroidism was the most common phenotype (n = 8, 50.0%). The vast majority of patients (63.5%) who presented with a thyroid event were diagnosed after ALE infusions. Significant thyroid eye disease requiring operative intervention was diagnosed in one patient (female, 42 years old). No thyroid dysfunction was diagnosed after CLA tablets were given. One patient (2.7%) with autoimmune thrombocytopenia was diagnosed after AHSCT.
The rates of ADs development did not differ between the sexes (Chi 2 = 0.957, p > 0.05), and age and treatment duration had no effect on AD frequency (p > 0.05). Patients with AD had a shorter disease duration than patients without AD did: 6.1 ± 3.5 years vs. 8.1 ± 6.0 years (p = 0.032).
The proportions of patients with secondary ADs (in black) among the total number of patients (in grey) for whom follow-up data are available per year after treatment with AHSCT, ALE and CLA are shown in Fig. 1 , Fig. 2 and Fig. 3 , respectively. The mean time to AD was 26.9 ± 13.9 months from initial treatment (range, 4–48 months). The shortest time to AD was in female patients who developed autoimmune thrombocytopenia at month 4 after AHSCT. The longest time to AD was 48 months in another female who developed hyperthyroidism after AHSCT. AD’s were more common between 37 and 60 months after AHSCT (with the exception of one thrombocytopenia patient), more frequent between 12 and 36 months after ALE and more common in the first 24 months after CLA treatment. No patients with ADs were identified after 60 months, although only 34 patients (19.0%) had longer follow-up.
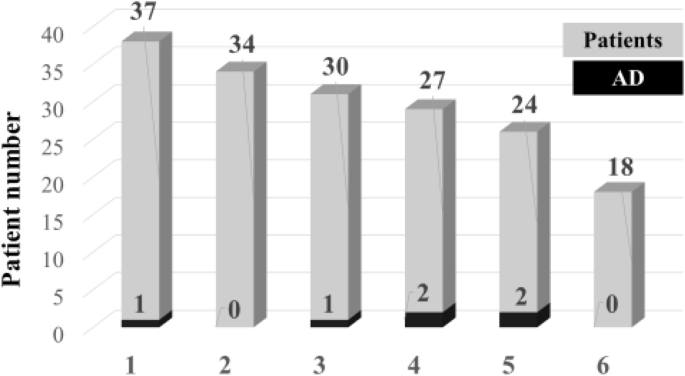
Occurrence of secondary AD per year of follow-up in MS patients treated with AHSCT. 1,—up to 12 months; 2,—13 −24 months; 3,—25–36 months; 4,—37–48 months; 5,—49–60 months; 6,—> 60 months. AD,—autoimmune disorder; AHSCT,—autologous haematopoietic stem cell transplantation. The number of patients with AD is shown in black relative to the total number of treated patients in grey.

Occurrence of secondary AD per year of follow-up in MS patients treated with ALE. 1,—up to 12 months; 2,—13 −24 months; 3,—25–36 months; 4,—37–48 months; 5,—49–60 months; 6,—> 60 months. AD,—autoimmune disorder; ALE,—alemtuzumab. The number of patients with AD is shown in black relative to the total number of treated patients in grey.

Occurrence of secondary AD per year of follow-up in MS patients treated with CLA. 1,—up to 12 months; 2,—13–24 months; 3,—25–36 months; 4,—37–48 months; 5,—49–60 months; 6,—> 60 months. AD,—autoimmune disorder; CLA,—cladribine. The number of patients with AD is shown in black relative to the total number of treated patients in grey.
Here, we report our single-centre experience of autoimmunity with 179 MS patients treated with IRT. We included patients treated with IRT who had more immune system depletion effects and no continuous immunosuppression effects. This is the first study describing the development of secondary autoimmune complications after all IRT in MS patients and comparing the differences between them.
First, demographic and disease characteristics (age, sex and disease duration) did not differ among the three groups in our study; however, the disease activity characteristic of the groups were different: relapse rate, disability level, and increased activity were observed in patients treated with AHSCT, whereas the lowest activity was observed in patients treated with CLA tablets. Additionally, more patients with higher efficacy of DMT were in the ALE and AHSCT groups. In most countries, CLA tablets are considered a reasonable option for first- or second-line therapy in patients with MS, whereas ALE and AHSCT are generally reserved for patients who respond poorly to second-line therapy 39 , 40 , 41 , 42 . Patients with higher disease activity were treated with higher-efficacy DMT, as confirmed by our results.
The greater number of patients who developed ADs after IRT were patients treated with ALE. The majority of ADs that developed after IRT were of thyroid origin, mostly after ALE infusion. AD outcomes in the ALE group are comparable with previous results from multicentre studies in Europe and smaller datasets 10 , 11 , 25 , 26 , 27 , 28 . The occurrence of thyroid dysfunction after the second course of ALE (41% of patients) was the same as that reported in previous studies 25 , 26 , 28 , 43 , 44 . In our cohort, the median follow-up in the ALE group was 61.1 ± 20.1 months, which was slightly greater than that reported in most previous studies 25 , 26 , 29 . Therefore, we reported the same occurrence of ADs with longer follow-up. Additionally, the highest rate of ADs in our study was observed during the second and third years after ALE infusions, so longer follow-up does not predict a higher incidence of ADs. For these reasons, 48-month clinical and biological monitoring is recommended after the last ALE infusion 45 .
New ADs were diagnosed in 16.2% of patients from week 4 up to 5 years after AHSCT in our study. The previously published occurrence of posttransplant autoimmune diseases ranges from 10% to 20 – 23% 18 , 19 , 46 , 47 . Several theories have proposed concerning the different rates of autoimmunity after AHSCT. The main focus in recent years has been on the different types of conditioning regimens used before AHSCT. The increased risk of autoimmune disease development was increased by the use of ALE instead of anti-thymocyte globulin (ATG) in the conditioning regimen 19 , 46 , 47 , 48 . ATG was used in our patients, but the occurrence of AD after AHSCT was relatively high. The lower number of patients could have had an impact on the relatively greater occurrence of AD after transplantation. All autoimmune thyroid disorders in the posttransplant patients in our study were diagnosed later than they were diagnosed after ALE infusions, possibly because de novo development of T cell can be delayed up to several years after T-cell depletion during AHSCT, and secondary AD can be diagnosed later through follow-up 18 , 49 .
The lowest occurrence of ADs in our study was associated with CLA treatment. These data are comparable to world data 37 ; only one case of autoimmune glomerulonephritis was published after CLA treatment 38 . In our cohort, two new autoimmune diseases, vitiligo and psoriasis, were diagnosed after CLA in the early follow-up. Our cases are the first to show new autoimmune disease development after the administration of CLA tablets. The time of occurrence of AD in our CLA patients was also quite early: the first signs of the disease appeared at months 10 and 13 after the first CLA course. The lower occurrence of ADs after CLA than after ALE and AHSCT are likely due to the different immune reconstitution effect intensities. CLA is thought to deplete primarily memory B cells and effector T cells, but it spares naive T cells and long-lived memory T cells to a greater degree than ALE does 11 . On the other hand, the depletion of lymphocytes is more profound, and the repletion of T- cells is slower after ALE than after CLA 1 .
The most common AD after IRT in our study was thyroid disorder, mostly after ALE and AHSCT, and the rates of AD did not differ based by sex or age among our patients. Although older age and female sex are known risk factors for autoimmune thyroid disease in general population studies 50 , they were not associated with the development of AD in our study. The same results in terms of age and sex after IRT were also published by Cossburn et al. 29 ; however, autoimmunity was assessed only after ALE. Our results and those of previous studies support the hypothesis that the mechanism by which autoimmune thyroid disorder occurs after ALE and AHSCT differs from that in the general population.
This study has several limitations, as we included fewer patients treated with ALE and AHSCT than with CLA; however, the follow-up was shorter in CLA patients than in ALE and AHSCT patients. The lower number of patients and the shorter follow-up period could have biased the study results. Additionally, it was descriptive single-centre study with no possibility of comparing the rate of occurrence of autoimmunity between different treatments. Furthermore, cladribine-treated patients had significantly lower disability rates and fewer previously used disease-modifying drugs, which might be another confounder. A greater number of drugs used in the past has a greater impact on the immune system and increases the likelihood of potential side effects. The key strength of our study lies in the fact that it is based on real-world clinical data prospectively collected by the same medical team at the same academic hospital centre, with criteria and goals for treatment remaining constant throughout the entire study period.
A single-centre retrospective noninterventional case series study using real-world data was conducted at Vilnius University Hospital Santaros Klinikos (VUHSK), Lithuania. The Lithuanian Bioethics Committee approved the study in 2011 (2011–01–27 Nr: L-12–01/2), the permission to continue the study was granted by the Lithuanian Bioethics Committee in 2018 (2018–02–22 Nr: 6B-18–41). A total of 179 MS patients treated with immune reconstitution therapy (AHSCT, ALE or CLA) between 2014 and 2023 were enrolled in the study. All methods were performed in accordance with the relevant guidelines and regulations. All patients signed the informed consent form for the collection of data and its use for research purposes.
Data were systematically acquired from electronic patient records. Patients’ electronic health records were reviewed through a nationwide system that includes all patients’ visits in all health care settings. Immune reconstitution treatment decisions were based on the clinical and radiological judgement of the neurology team at VUHSK. Patients were treated with two annual courses of ALE or CLA 3 , 4 . A medium-intensity conditioning regimen with cyclophosphamide and antithymocyte globulin was used for all transplant patients. Owing to the routine monitoring requirements, follow-up visits in ALE patients were performed every month five years from the first infusion and then every three months. Patients after the CLA course were monitored monthly three months after the treatment course and every three month after that. Patients after AHSCT were monitored monthly six months after the treatment and every three months thereafter.
The primary outcome measure of the study was the proportion of patients who developed new ADs. New AD was recognized to be caused by IRT in those cases when it was not present in the patient prior to therapy exposure. ADs were diagnosed on the basis of symptoms, medical history, blood tests, and,in some cases, imaging tests and biopsies. The diagnoses of all thyroid autoimmune diseases were confirmed by an endocrinologist upon endocrinological assessment, cytopenia was confirmed by a hematologist, and skin diseases were confirmed by a dermatologist upon dermatological assessments. The timing of an AD was defined as the day of first symptom manifestation or when the disorder was first recognized by a health care professional.
Statistical methods
The data were analysed via the statistical software package SPSS (version 23.0 for Windows). Continuous variables are reported as medians and ranges or means and standard deviations, whereas categorical variables are reported as absolute numbers and percentages of total patients. Kruskal‒Wallis test was used to determine the differences between the means of the three treatment groups. Categorical variables were analysed via the chi-square test or Fisher’s exact test. To check the normality of the distribution of quantitative variables, the Shapiro‒Wilk test was used. A significance level of p < 0.05 was considered to indicate statistical significance.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Sorensen, P. S. & Sellebjerg, F. Pulsed immune reconstitution therapy in multiple sclerosis. Ther. Adv. Neurol. Disord. 28 (12), 1756286419836913 (2019).
Article Google Scholar
Karussis, D. & Petrou, P. Immune reconstitution therapy (IRT) in multiple sclerosis: the rationale. Immunol. Res. 66 (6), 642–648 (2018).
Article CAS PubMed MATH Google Scholar
Lemtrada product information. European Medicines Agency. 2023. https://www.ema.europa.eu/en/documents/product-information/lemtrada-epar-product-information_en.pdf Accessed on 06. June, 2024.
Mavenclad product information. European Medicines Agency. https://www.ema.europa.eu/en/documents/product-information/mavenclad-epar-product-information_en.pdf Accessed on 06. June, 2024.
Snowden, J. A. et al. Autologous haematopoietic stem cell transplantation (aHSCT) for severe resistant autoimmune and inflammatory diseases - a guide for the generalist. Clin. Med. (Lond.). 18 (4), 329–334. https://doi.org/10.7861/clinmedicine.18-4-329 (2018).
Article PubMed PubMed Central Google Scholar
Lünemann, J. D., Ruck, T., Muraro, P. A., Bar-Or, A. & Wiendl, H. Immune reconstitution therapies: concepts for durable remission in multiple sclerosis. Nat. Rev. Neurol. 16 (1), 56–62 (2020).
Article PubMed Google Scholar
AlSharoqi, I. A. et al. Immune reconstitution therapy or continuous immunosuppression for the management of active relapsing-remitting multiple sclerosis patients? A narrative review. Neurol. Ther. 9 (1), 55–66 (2020).
Collins, F., Kazmi, M. & Muraro, P. A. Progress and prospects for the use and the understanding of the mode of action of autologous hematopoietic stem cell transplantation in the treatment of multiple sclerosis. Expert Rev. Clin. Immunol. 13 (6), 611–622 (2017).
Raiola, A. M., Ghiso, A., Gambella, M. & Angelucci, E. The HSCT procedure (II): Conditioning, hematopoietic stem cell infusion, supportive care, and monitoring. Handb. Clin. Neurol. 202 , 117–134 (2024).
Coles, A. J. Alemtuzumab therapy for multiple sclerosis. Neurotherapeutics 10 (1), 29–33 (2013).
Costelloe, L., Jones, J. & Coles, A. Secondary autoimmune diseases following alemtuzumab therapy for multiple sclerosis. Expert Rev. Neurother. 12 (3), 335–341 (2012).
Article CAS PubMed Google Scholar
Sherer, Y. & Shoenfeld, Y. Autoimmune diseases and autoimmunity post-bone marrow transplantation. Bone Marrow Transpl. 22 , 873–881 (1998).
Article CAS MATH Google Scholar
Daikeler, T. & Tyndall, A. Autoimmunity following haematopoietic stem-cell transplantation. Best Pract. Res. Clin. Haematol. 20 , 349–360 (2007).
Bohgaki, T., Atsumi, T. & Koike, T. Autoimmune disease after autologous hematopoietic stem cell transplantation. Autoimmun. Rev. 7 (3), 198–203 (2008).
Au, W. Y. et al. Autoimmune thyroid dysfunction after hematopoietic stem cell transplantation. Bone Marrow Transplant. 35 (4), 383–388 (2005).
Article MathSciNet CAS PubMed Google Scholar
Burt, R. K. et al. New autoimmune diseases after autologous hematopoietic stem cell transplantation for multiple sclerosis. Bone Marrow Transplant. 56 (7), 1509–1517 (2021).
Hequet, O. et al. Autoimmune thrombocytopenic purpura after autologous stem cell transplantation. Bone Marrow Transplant. 32 (1), 89–95 (2003).
Daikeler, T. et al. Secondary autoimmune diseases occurring after HSCT for an autoimmune disease: a retrospective study of the EBMT Autoimmune Disease Working Party. Blood 118 (6), 1693–1698 (2011).
Loh, Y. et al. Development of a secondary autoimmune disorder after hematopoietic stem cell transplantation for autoimmune diseases: role of conditioning regimen. Blood 109 (6), 2643–3548 (2007).
Alping, P., Burman, J., Lycke, J., Frisell, T. & Piehl, F. Safety of alemtuzumab and autologous hematopoietic stem cell transplantation compared to noninduction therapies for multiple sclerosis. Neurology 96 (11), e1574–e1584 (2021).
Article CAS PubMed PubMed Central Google Scholar
von Essen, M. R., Chow, H. H., Holm Hansen, R., Buhelt, S. & Sellebjerg, F. Immune reconstitution following alemtuzumab therapy is characterized by exhausted T cells, increased regulatory control of proinflammatory T cells and reduced B cell control. Front. Immunol. 6 (14), 1249201 (2023).
Ruck, T. et al. Alemtuzumab-induced immune phenotype and repertoire changes: implications for secondary autoimmunity. Brain 145 (5), 1711–1725 (2022).
Article PubMed PubMed Central MATH Google Scholar
Jones, J. L. et al. IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H). J. Clin. Invest. 119 (7), 2052–2061 (2009).
CAS PubMed PubMed Central MATH Google Scholar
Ghalamfarsa, G. et al. IL-21 and IL-21 receptor in the immunopathogenesis of multiple sclerosis. J. Immunotoxicol. 13 (3), 274–285 (2016).
Pariani, N. et al. Alemtuzumab-induced thyroid dysfunction exhibits distinctive clinical and immunological features. J. Clin. Endocrinol. Metab. 103 , 3010–3018 (2018).
Havrdova, E. et al. Alemtuzumab CARE-MS I 5-year follow-up. Neurology 89 , 1107–1116 (2017).
Article CAS PubMed PubMed Central MATH Google Scholar
Muller, I. et al. 2019 European thyroid association guidelines on the management of thyroid dysfunction following immune reconstitution therapy. Eur. Thyroid. J. 8 , 173–185 (2019).
Scappaticcio, L. et al. Alemtuzumab-induced thyroid events in multiple sclerosis: a systematic review and meta-analysis. J. Endocrinol. Invest. 43 , 219–229 (2020).
Cossburn, M. et al. Autoimmune disease after alemtuzumab treatment for multiple sclerosis in a multicenter cohort. Neurology 77 (6), 573–579 (2011).
Decallonne, B. et al. Thyroid disorders in alemtuzumab-treated multiple sclerosis patients: a Belgian consensus on diagnosis and management. Acta Neurol. Belg. 118 , 153–159 (2018).
Cuker, A. et al. Immune thrombocytopenia in alemtuzumab-treated MS patients: Incidence, detection, and management. Mult. Scler. 26 (1), 48–56 (2020).
Sarvepalli, D., Rashid, M. U., Ullah, W., Zafar, Y. & Khan, M. Idiopathic thrombocytopenic purpura: A rare syndrome with alemtuzumab, review of monitoring protocol. Cureus 11 (9), e5715 (2019).
PubMed PubMed Central MATH Google Scholar
White, E., Watson, A., Holian, J., McGuigan, C. & O’Riordan, S. Glomerulonephritis with positive anti-glomerular basement membrane antibodies following alemtuzumab treatment. Ir. Med. J. 113 (3), 41 (2020).
CAS PubMed Google Scholar
Almuhaiteeb, A., Alkeay, K. & Altaleb, A. Glomerulonephritis after alemtuzumab treatment for multiple sclerosis: A report of two cases. Glomerular Dis. 4 (1), 84–90 (2024).
PubMed PubMed Central Google Scholar
Tzartos, J. S. et al. Autoimmune hemolytic anemia, demyelinating relapse, and AQP1 antibodies after alemtuzumab infusion. Neurol. Neuroimmunol. Neuroinfamm. 7 (3), e711 (2020).
Alnahdi, M. A., Aljarba, S. I. & Al Malik, Y. M. Alemtuzumab-induced simultaneous onset of autoimmune haemolytic anaemia, alveolar haemorrhage, nephropathy, and stroke: A case report. Mult. Scler. Relat. Disord. 41 , 102141 (2020).
Cook, S. et al. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: an integrated analysis. Mult. Scler. Relat. Disord. 29 , 157–167 (2019).
Article PubMed MATH Google Scholar
Schönfelder, K. et al. Autoimmune glomerulonephritis in a multiple sclerosis patient after cladribine treatment. Mult. Scler. 27 (12), 1960–1964 (2021).
López-Real, A. M. et al. Alemtuzumab treatment in real clinical practice: Experience in a multicenter cohort. Mult. Scler. Relat. Disord. 75 , 104762 (2023).
Lambe, T. et al. Cladribine tablets for the first-line treatment of relapsing-remitting multiple sclerosis: An evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics 37 (3), 345–357 (2019).
Zanetta, C. et al. Effectiveness and safety profile of cladribine in an Italian real-life cohort of relapsing-remitting multiple sclerosis patients: a monocentric longitudinal observational study. J. Neurol. 270 (7), 3553–3564 (2023).
Ross, L. A., Stropp, L. M. & Cohen, J. A. Autologous hematopoietic stem cell transplantation to treat multiple sclerosis. Neurol. Clin. 42 (1), 165–184 (2024).
Tuohy, O. et al. Alemtuzumab treatment of multiple sclerosis: long-term safety and efficacy. J. Neurol. Neurosurg. Psychiatry 86 , 208–215 (2015).
Daniels, G. H. et al. Alemtuzumab-related thyroid dysfunction in a phase 2 trial of patients with relapsing-remitting multiple sclerosis. J. Clin. Endocrinol. Metab. 99 , 80–89 (2014).
Berger, T. et al. Alemtuzumab Use in Clinical Practice: Recommendations from European Multiple Sclerosis Experts. CNS Drugs 31 (1), 33–50 (2017).
Willison, A. G., Ruck, T., Lenz, G., Hartung, H. P. & Meuth, S. G. The current standing of autologous haematopoietic stem cell transplantation for the treatment of multiple sclerosis. J. Neurol. 269 (7), 3937–3958 (2022).
Burt, R. K., Han, X., Quigley, K., Helenowski, I. B. & Balabanov, R. Real-world application of autologous hematopoietic stem cell transplantation in 507 patients with multiple sclerosis. J. Neurol. https://doi.org/10.1007/s00415-021-10820-2 (2021).
Haussler, V. et al. aHSCT is superior to alemtuzumab in maintaining NEDA and improving cognition in multiple sclerosis. Ann. Clin. Transl. Neurol. 8 (6), 1269–1278 (2021).
Farge, D. et al. Analysis of immune reconstitution after autologous bone marrow transplantation in systemic sclerosis. Arthritis Rheum. 52 (5), 1555–1563 (2005).
Mammen, J. S. R. & Cappola, A. R. Autoimmune thyroid disease in women. JAMA. 325 (23), 2392–2393 (2021).
Download references
Author information
Authors and affiliations.
Clinic of Neurology and Neurosurgery, Institute of Clinical Medicine, Faculty of Medicine, Vilnius University, Vilnius, Lithuania
Nataša Giedraitienė, Rasa Kizlaitienė & Gintaras Kaubrys
You can also search for this author in PubMed Google Scholar
Contributions
N.G. Conception and design of the study, acquisition of the data, analysis and interpretation of the data, drafting of the manuscript. R.K. Concept of the study, revision of the manuscript for scientific accuracy. G.K. Overall revision for accuracy of the data, tables and figures, scientific accuracy of the manuscript. All authors reviewed the manuscript.
Corresponding author
Correspondence to Nataša Giedraitienė .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Giedraitienė, N., Kizlaitienė, R. & Kaubrys, G. New autoimmune disorder development after immune reconstitution therapy for multiple sclerosis. Sci Rep 14 , 30991 (2024). https://doi.org/10.1038/s41598-024-82196-y
Download citation
Received : 28 September 2024
Accepted : 03 December 2024
Published : 28 December 2024
DOI : https://doi.org/10.1038/s41598-024-82196-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Relapsing multiple sclerosis
- Immune reconstitution therapy
- Autoimmune disorder
- Autologous haematopoietic stem cell transplantation
- Alemtuzumab
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Accessibility
- What is Multiple Sclerosis?
- Types of MS
- HELPFUL LINKS
- MS Resources
- Newly Diagnosed Guide
- The Latest Research
- Read the Blog
- Find Support
- LIVING WITH MS
- Health and Wellness Therapies
- Treatments in Development
- Participate in Research
- FIND HELP & GET SUPPORT
- Find Support Programs
- 1:1 Peer Support
- MS Support Groups
- Quality of Life Program
- CONNECT WITH COMMUNITY
- Find Community Events
- Volunteer with Us
- THE LATEST MS RESEARCH
- MS CANADA RESEARCH
- Our Research Program
- Information for Researchers
- UPCOMING EVENTS
- We Challenge MS
- Find More Community Events
- GIVE TIME, ACTION, FUNDS
- Advocate for Change
- Ways to Donate
- Add a Legacy Gift
- THE ORGANIZATION
About MS Canada
- Careers with MS Canada
- LET'S CONNECT
- News & Publications
About Multiple Sclerosis (MS)
Learn more to understand MS.
Chat with an MS Navigator
Managing Multiple Sclerosis
MS is not curable, but it is manageable through treatments and lifestyle adjustments.
Multiple Sclerosis Support
Whether you’re seeking support for yourself, trying to find peers in the MS community, or are a caregiver, the MS Canada team has programs & services for you.
Leading MS Research
We’re committed to research that could bring the MS community closer to a cure for multiple sclerosis.
Become a Research Participant
Join our efforts against MS
By fundraising, advocating, volunteering and more, you can support those living with MS and the researchers dedicated to uncovering the next breakthrough treatments.
Donate to MS Canada
We provide services to people living with MS and their families while advocating for change and funding research to end this disease.
Research Advances that are Paving the Way to a Future Without MS
Here’s what we achieved together in 2024.

Reflecting on this past year in research, we’re proud to highlight the great work and progress that’s advancing our understanding of multiple sclerosis (MS). Advances in research are only possible through the valuable contributions and generous support from the amazing MS community, including people living with MS, event participants, volunteers, fundraisers, donors, research partners, researchers, clinicians, and many others. The unwavering collective support and dedication are moving us closer towards a future free of MS!
Below we share some of the exciting research from this year, including Canadian MS research achievements on the global stage, our support in strengthening connections within the MS community, and new research within our impact goals.
We drive the momentum of MS research forward by investing in promising work across our impact goals, which are a roadmap for our journey towards a world without MS. Our impact goals focus on advancing treatment and care, to help people on their unique MS journey; enhancing well-being by ensuring that people living with MS have access to opportunities and supports; understanding the mechanisms of MS progression to stop the disease in its tracks; and preventing MS before it can even begin.
Here are some of the research advances in the past year across the following impact goal areas.
Advancing Treatment and Care
- The First Canadian Best Practice Guidelines for MS Rehabilitation . This year, we partnered with Saskatchewan Health Research Foundation (SHRF) to support Dr. Sarah Donkers ’ (University of Saskatchewan) work on creating a series of clinical guidelines or recommendations for MS rehabilitation and symptom management, based on best available evidence and input from experts and people with MS. These guidelines will be a helpful resource for improving the quality of care and health outcomes for people living with MS. Read more about this study .
- A Novel Intervention to Improve Balance in People with MS. Research led by Dr. Aimee Nelson (McMaster University) will test a unique training method for improving balance in people with MS. Study participants will be asked to control a video game using signals from their leg and arm muscles while standing with or without assistance. This research could help reverse the loss of function and encourage physical activity in people living with MS. Read more about this study .
Understanding and Halting Disease Progression
Targeting Neurodegeneration in MS. Research led by Dr. Michael Levin (University of Saskatchewan) found that a dysfunction in a factor called ‘hnRNP A1’ is associated with neurodegeneration in MS (read more about the findings ). In a new study funded in partnership with SHRF , Dr. Levin and his team will develop small molecule treatments that can rescue the function of hnRNP A1 to reduce neurodegeneration in animal models of MS. This study could help find new treatments to test in early clinical trials for people with progressive MS. Learn more about this study .
- A Promising Candidate to Promote Neuroprotection and Repair Processes in MS. Dr. Soheila Karimi ’s (University of Manitoba) research, which we funded, found a potential treatment, called Neuregulin-1, that could help repair the protective covering of nerve fibres and prevent further damage. In this new study, the research team will use mice with MS-like disease to test how effective Neuregulin-1 is as a treatment that protects nerves and helps repair them in progressive MS, particularly in improving cognition, movement, and sensation (feeling). This research will be very important for future development of new treatments for progressive MS. Read more about this study .
Preventing MS
An Opportunity for Earlier Detection and Prevention of MS. For many years we’ve supported Dr. Helen Tremlett ’s (University of British Columbia) work on characterizing the MS prodrome, which involves studying the early signs or symptoms that occur years before MS is officially diagnosed. A recent report from Dr. Tremlett’s team found that people with MS often needed to use healthcare services to support their mental health needs and gastrointestinal-related problems in the five years before their MS diagnosis. This is very important work as a better characterization of the MS prodrome may allow for earlier disease detection and treatment to change the disease course or even prevent MS. Learn more about this study .
- Uncovering Genetic Mechanisms in MS . Dr. Parisa Shooshtari (Western University) will combine her expertise in genetics, immune system function, and statistics to study how changes in the genes of certain immune cells might cause MS. This is exciting as we hope the findings from this research will provide a better understanding of the early events that lead to MS, laying the groundwork for future studies. Read more about this study .
Maximizing Impact Through Global Partnerships
Working with partners around the world has helped us make a bigger difference and speed up important research that benefits people with MS, by sharing resources, knowledge, and experience.
- As a founding and managing partner of the International Progressive MS Alliance (the Alliance), we work closely with global partners to tackle challenges in progressive MS. In the past year, the Alliance has invested over 4.6 million euros on research initiatives aimed to address the urgent need for new, effective treatments for people living with MS. Several Canadian researchers are among the successful recipients of these international awards!
![latest ms research [Recipients of the Alliance awards – Drs. Jennifer Gommerman, Lara Pilutti, and Gabrielle Macaron]](https://mscanada.ca/sites/default/files/styles/original/public/images/2024-12/1-Alliance%20Awardees.jpg?itok=Mkd3c0sH)
Dr. Jennifer Gommerman (University of Toronto) received one of six Challenges in Progressive MS Awards for her work in understanding how the immune system contributes to cognitive dysfunction in progressive MS (read more about this study ). Drs. Lara Pilutti (University of Ottawa) and Gabrielle Macaron (Centre Recherche Centre Hospitalier Université de Montreal) received two of nine Innovations in Well-Being Awards . Dr. Pilutti is looking at rehabilitation approaches to restore cognition and motor function in people with progressive MS (read more about this study ). Dr. Macaron is looking at better ways to detect cognitive impairments in people with progressive MS.
See the full list of Alliance projects.
- We’re committed to working with MS organizations from around the world to pursue research with the greatest potential to stop new disease activity, restore function and repair damage, and end MS through prevention, as highlighted in the latest version of the Pathways to Cures Roadmap . The updated Roadmap takes into account new scientific advances in MS as well as the current funding landscape of MS research to find the most important areas to focus on and boost our combined efforts. Read more about the updated research Roadmap .
Strengthening Community Connections
We strive to provide opportunities for our MS community to connect, share ideas, and work together to make a positive impact.
- Creating Strong Connections Between People With MS. Founded by Drs. Sarah Donkers and Katherine Knox (University of Saskatchewan), “ Neurosask: Active and Connected ” is a virtual program providing physiotherapy-guided exercise, expert information on health and wellness, and social connection for people with neurological conditions like MS. As a supporter of this program, we’re thrilled to hear how much it has impacted our MS community! Participants shared that the program helped them feel more connected to others and improved their physical skills, everyday function, and quality of life. Read more about what participants had to say about their Neurosask experience .
“I love that the active sessions are live and the exercises are slightly adjusted each time so that we can work our body in different ways – they are really engaging and fun! I’m encouraged to attend each session and get valuable exercises completed. I also love the connect sessions as they provide a variety of useful information. Neurosask is such a wonderful program and I’m so grateful that it exists.” - NeuroSask participant, living with MS
- Providing a Voice for All People Affected by MS. Our Community Representative program was created in 2012, with the goal of involving people affected by MS in our research review process. Through this program, we give people affected by MS a platform to voice their opinions on MS research priorities and make sure that fundraising dollars are spent on impactful research that will ultimately improve the lives of people with MS. To-date, we’ve had over 65 Community Representatives from across Canada – a talented group of community members who have played a key role in shaping the research we fund. Read more about our Champions of Impact !
![latest ms research [Geoff Robinson, male wearing a pink checkered shirt, smiling against tan background]](https://mscanada.ca/sites/default/files/styles/original/public/images/2024-11/Geoff-community-rep.png?itok=H1RG-lC0)
“I loved participating in lively discussions that lay the groundwork for developments in MS management. It was great to see the level of engagement the researchers bring to the table.”
- Geoff Robinson, Community Representative
Bringing the MS Research Community Together. We also hosted our 6 th endMS Conference – the largest scientific meeting focused on MS research in Canada! This conference brought the MS research community together, where they shared the latest in research to support the development of new ideas that have the potential to accelerate research in MS.
Over 320 people attended from across Canada and the world, including researchers, clinicians, trainees, and people affected by MS. The conference showcased 45 scientific speakers who shared cutting-edge research on topics like the MS prodrome , new targets for repair and remyelination, advances in imaging techniques, diet, physical activity in MS, and much more.

“When you’re diagnosed with MS you have no idea that beyond your immediate support and your medical support, that there’s a whole community of people that don’t know you at all, but they have your very best interests at heart and are devoting their life’s work…to making the world a better place for you.”
- Sue Whittaker, MS Ambassador and Community Representative
This conference was a success because of the leadership from our scientific co-chairs, the support from the scientific organizing committee members, the exceptional speakers, engaged participants, and dedicated sponsors – thank you all!

Empowering the Next Generation of MS Leaders
We support the growth of MS research by training the next generation of talented researchers and future leaders. We’re committed to supporting researchers – whether they are in the early stages of graduate school or leading research as an independent investigator.
Through our annual research competition, we provided 57 endMS Personnel awards to support trainees who are completing their postdoctoral work or doctoral studies in the MS field. We also funded some of the most promising up-and-coming researchers who are pushing MS research forward in Canada. Read more about some of the early career researchers in the MS field.
![latest ms research [Participants of the 2024 endMS Summer School including staff, trainees, and researchers]](https://mscanada.ca/sites/default/files/styles/original/public/images/2024-12/5-endMS%20Summer%20School.jpg?itok=sConPGiH)
- We’re proud to support the endMS National Training Program , led by Dr. Christina Wolfson (McGill University), which offers a variety of educational and networking opportunities for trainees to improve their understanding and skills in MS research. An example of this opportunity is the endMS Summer School, which was hosted by Drs. Soheila Karimi and Chase Figley at the University of Manitoba and Manitoba MS Research Centre in Winnipeg. This year’s Summer School focused on mechanisms of disease and repair strategies, and was attended by 88 participants, including graduate students, postdoctoral and clinical fellows, speakers and panelists from across Canada. Read more about the endMS Summer School .
![latest ms research [A magnetic resonance imaging machine at the Manitoba MS Research Centre]](https://mscanada.ca/sites/default/files/styles/original/public/images/2024-12/6-endMS%20Summer%20School.jpg?itok=MgZBMHnm)
- We provided Education Awards to 9 Canadian trainees attending the IMSVISUAL Summer School . The intensive two-day summer school was a great opportunity for trainees to improve their understanding of how MS affects the visual system (the parts of the body that help us see) through a series of educational sessions and hands-on workshops.
We are truly grateful to the MS researchers who are expanding our understanding of the disease, and to the MS community whose support makes this progress possible. Together, we can push for progress that will not only improve the lives of people living with MS today, but also shape the future of MS treatment and care for the future!
To stay updated on MS research, read the latest in research news and subscribe to our quarterly Research in Action newsletter. You can also check out Your Guide to Exploring MS Research for ways to stay informed!
More Stories You May Like

Champions of Impact: Inside our Community Representative Program
![latest ms research [Site du congrès de l’ECTRIMS où l’on peut voir des banderoles de bienvenue, des kiosques d’entreprise et des personnes participant à l’événement.]](https://mscanada.ca/sites/default/files/styles/card_default/public/images/2024-11/Picture3_1.jpg?itok=BVqyQWkh)

Frontiers in MS Research: ECTRIMS 2024 Highlights
- Log in to post comments
Sign up for our e-newsletter
Health Conditions
- Breast Cancer
- Cancer Care
- Caregiving for Alzheimer's Disease
- Chronic Kidney Disease
- Chronic Obstructive Pulmonary Disease (COPD)
- Digestive Health
- Heart Health
- Mental Health
- Multiple Sclerosis (MS)
- Rheumatoid Arthritis (RA)
- Sleep Health
- Type 2 Diabetes
- Weight Management
Condition Spotlight
Wellness Topics
- Mental Well-Being
- Sexual Health
- Vitamins and Supplements
- Women's Wellness
Product Reviews
- At-Home Testing
- Men's Health
- Women's Health
Featured Programs
- Video Series
- Pill Identifier
- Crohn’s and Ulcerative Colitis Essentials
- Diabetes Nutrition
- High Cholesterol
- Taming Inflammation in Psoriasis
- Taming Inflammation in Psoriatic Arthritis
Newsletters
- Anxiety and Depression
- Nutrition Edition
- Wellness Wire
Lifestyle Quizzes
- Find a Diet
- Find Healthy Snacks
- How Well Do You Sleep?
- Are You a Workaholic?
Find Your Bezzy Community
Bezzy communities provide meaningful connections with others living with chronic conditions. Join Bezzy on the web or mobile app.

Follow us on social media
Can't get enough? Connect with us for all things health.

How Close Are We to a Cure for Multiple Sclerosis?

New disease-modifying therapies for multiple sclerosis can help slow down disease progression. Some experimental therapies also show promise in treating the disease.
There’s currently no cure for multiple sclerosis (MS), but there are several treatment options that can help manage it. In recent years, new medications have become available to help slow the progression of the disease and relieve symptoms.
Researchers are continuing to develop new treatments and learn more about the causes and risk factors of this disease.
Read on to learn about some of the latest treatment breakthroughs and promising avenues of research.
New disease-modifying therapies

Disease-modifying therapies (DMTs) are the main group of medications used to treat MS. To date, the Food and Drug Administration (FDA) has approved more than a dozen DMTs for different types of MS.
Most recently, the FDA has approved:
- Ocrelizumab (Ocrevus): This drug treats relapsing forms of MS and primary progressive MS (PPMS). It’s the first DMT to be approved to treat PPMS and the only one approved for all four types of MS.
- Fingolimod (Gilenya) : This drug treats pediatric MS. It was already approved for adults and, in 2018, became the first DMT to be approved for children .
- Cladribine (Mavenclad) : This drug is approved to treat relapsing-remitting MS (RRMS) and active secondary progressive MS (SPMS).
- Siponimod (Mayzent): This drug is approved to treat RRMS, active SPMS, and clinically isolated syndrome (CIS). In a phase 3 clinical trial , siponimod effectively reduced the rate of relapse in people with active SPMS. Compared with a placebo, it cut the relapse rate in half.
- Ponesimod (Ponvory): This FDA-approved drug has been shown to reduce annual relapses for relapsing types of MS by 30.5% when compared with teriflunomide (Aubagio).
- Ublituximab (Briumvi): This drug was approved by the FDA to treat RRMS, SPMS, and CIS. It is a monoclonal antibody given as an infusion.
While new treatments are continually being approved, some medications are being removed from pharmacy shelves. In March 2018, daclizumab (Zinbryta) was withdrawn from markets around the world due to reports of the drug potentially causing inflammatory brain disorders. This drug is no longer available to treat MS.
Experimental therapies
Several other medications are moving through the research pipeline. In recent studies, some of these medications have shown promise for treating MS.
- The results of a phase 2 clinical trial suggest that the drug ibudilast might help reduce the progression of MS. To learn more about this medication, the manufacturer plans to conduct a phase 3 clinical trial.
- The findings of a small 2017 study suggest that clemastine fumarate might help restore the protective coating around nerves in people with relapsing forms of MS. This oral antihistamine is currently available over the counter but not in the dose used in the clinical trial. More research is needed to study its potential benefits and risks for treating MS.
- Hematopoietic stem cell transplantation therapy is a promising new treatment for MS that’s currently being studied. It’s not currently approved in the United States, but interest is growing in the field, and it’s being evaluated in clinical trials .
Data-driven strategies to target treatments
Thanks to the development of new medications for MS, people have a growing number of treatment options to choose from.
To help guide their decisions, scientists are using large databases and statistical analyses to try to pinpoint the best treatment options for different people.
Eventually, this research might help those with MS learn which treatments are most likely to work.
Progress in gene research
To understand the causes and risk factors for MS, geneticists and other scientists are combing the human genome for clues.
Researchers have identified more than 200 genetic variants associated with MS. For example, a 2018 study by the International Multiple Sclerosis Genetics Consortium identified 4 new genes linked to the disease.
Eventually, findings like these might help scientists develop new strategies and tools to predict, prevent, and treat MS.
Studies of the gut microbiome
Scientists have also studied the role that bacteria and other microbes in our gut might play in the development and progression of MS. This community of bacteria is known as our gut microbiome.
Not all bacteria are harmful. In fact, many “friendly” bacteria live in our bodies and help regulate our immune system.
When the balance of bacteria in our bodies is off, it can lead to inflammation. This might contribute to the development of autoimmune diseases, including MS.
Research into the gut microbiome might help scientists understand why and how people develop MS. It could also pave the way for new treatment approaches, including dietary interventions and other therapies.
The takeaway
Scientists are continuously seeking new insight into the risk factors and causes of MS and potential treatment strategies.
Several new medications have been approved in recent years, and some have shown promise in clinical trials.
These advancements are helping to improve the health and well-being of the many people who live with this condition while bolstering hopes for a potential cure.
How we reviewed this article:
- Best available therapy versus autologous hematopoetic stem cell transplant for multiple sclerosis (BEAT-MS) (BEAT-MS). (2024). Daclizumab withdrawn from the market worldwide. (2018). https://clinicaltrials.gov/ct2/show/NCT04047628
- Disease modification: The benefits of and considerations for using disease-modifying therapies (DMTS) for multiple sclerosis. (n.d.). https://www.nationalmssociety.org/for-professionals/for-healthcare-professionals/managing-and-treating-ms/disease-modification#section-1
- Drug trials snapshot: Briumvi. (2023). https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-briumvi
- Drug trials snapshot: Ponvory. (2023). https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshot-ponvory
- FDA approves new drug to treat multiple sclerosis. (2018). https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treat-multiple-sclerosis
- FDA approves new oral drug to treat multiple sclerosis. (2019). https://www.fda.gov/news-events/press-announcements/fda-approves-new-oral-drug-treat-multiple-sclerosis
- FDA approves new oral treatment for multiple sclerosis. (2019). https://www.fda.gov/news-events/press-announcements/fda-approves-new-oral-treatment-multiple-sclerosis
- FDA expands approval of Gilenya to treat multiple sclerosis in pediatric patients. (2018). https://www.fda.gov/news-events/press-announcements/fda-expands-approval-gilenya-treat-multiple-sclerosis-pediatric-patients
- Fox RJ, et al. (2018). Phase 2 trial of ibudilast in progressive multiple sclerosis. https://www.nejm.org/doi/10.1056/NEJMoa1803583
- Green AJ, et al. (2017). Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): A randomised, controlled, double-blind, crossover trial. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(17)32346-2/fulltext
- Hecker M, et al. (2019). A genetic variant associated with multiple sclerosis inversely affects the expression of CD58 and microRNA-548ac from the same gene. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6382214/
- Henschke A, et al. (2021). Personalizing medicine and technologies to address the experiences and needs of people with multiple sclerosis. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8401762/
- International Multiple Sclerosis Genetics Consortium. (2018). Low-frequency and rare-coding variation contributes to multiple sclerosis risk. https://linkinghub.elsevier.com/retrieve/pii/S0092867418312613
- Kappos L, et al. (2018). Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): A double-blind, randomised, phase 3 study. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)30475-6/fulltext
- Kappos L, et al. (2021). Ponesimod compared with teriflunomide in patients with relapsing multiple sclerosis in the active-comparator phase 3 OPTIMUM study. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8008435/
- Kirby TO, et al. (2018). The gut microbiome in multiple sclerosis: A potential therapeutic avenue. https://www.mdpi.com/2076-3271/6/3/69/htm
Share this article
More in Find Your Fierce with MS
- Oral vs. Injectable MS Treatments: What’s the Difference?
- New Exercises and Activities to Try If You Have MS
- Learning to Advocate for Yourself with MS: A Guide
- How to Choose the Best MS Treatment for Your Lifestyle
Read this next
Multiple sclerosis can cause symptoms that may interfere with your sex drive and your enjoyment of sex. Learn tips to manage those symptoms and…
This short assessment can help determine whether your MS is well managed, or if there are signs that you should discuss with your doctor.
MS affects women and men, but there are some differences in how the condition tends to progress and how it affects quality of life. Read on to learn…
Discover how multiple sclerosis (MS) could affect pregnancy, and vice versa. Learn the facts on relapses, breastfeeding, what happens postpartum, and…
Staying active with MS has been a big challenge, but I've kept doing activities I love, such as running and weightlifting.

Top 10 MS news stories of 2024
The team at Multiple Sclerosis News Today has brought our audience the latest news about treatments, scientific research, and clinical trials in multiple sclerosis (MS) throughout 2024. Here is a list of the top 10 most-read articles we published this year. We look forward to continuing to serve…
Vascular age seen higher than chronological age in MS patients
People with multiple sclerosis (MS) show significant differences between actual age and vascular age, an indicator of heart and blood vessel health, even if they don’t have any cardiovascular disease, a study found. The difference became more pronounced with the presence of additional cardiovascular diseases, reaching a peak among…

Men who started on high-efficacy DMTs had fewer relapses: Analysis
Men with multiple sclerosis (MS) who started on high-efficacy disease-modifying therapies (DMTs) experienced significantly fewer relapses than those who started with moderate-efficacy DMTs, according to real-world claims analysis involving more than 10,000 U.S. patients. “No significant difference in relapse was found among females,” researchers wrote. “This observation highlights…

A comprehensive collective nouns list for multiple sclerosis
For someone like me, who is retired and has three preteen children, the days surrounding Christmas and New Year’s feel like a liminal space. With no school or job to be at and no real schedule to keep, the days seem to run together without anything to orient them.

It’s frustrating when heat aggravates my MS symptoms
Being born and raised in south-central Texas, I’m no stranger to the heat here that dominates most of the year. But even after 25 years of living in Texas, I don’t think I’m used to it. While some people embrace and enjoy warmer weather, my body rejects it altogether, especially…

Featured Column MS presents some of the toughest challenges, but don’t quit
When faced with the obstacle course that is MS, columnist Ben Hofmeister remembers his strategy while in the Army Special Forces: Don’t quit.

Your MS community
Visit the Multiple Sclerosis News Today forums to connect with others in the MS community.
Perspectives

With MS, I don’t want to lose control when I should give it away
My mom has been my advocate as i’ve been living with ms.

Among all my symptoms, MS has also granted me significant gifts

Changing neurologists yet again to treat my MS
Special collections.

Living Well With Multiple Sclerosis

Pain and Multiple Sclerosis

Sex, Intimacy, and Multiple Sclerosis
Subscribe to our newsletter.
Get regular updates to your inbox.
MS Research
The National MS Society is bringing the world together to cure MS for every single person — as fast as possible. Learn more about the most promising cures for MS.
More About MS Research

- 0 (Current Slide)
How You Can Support Research

Get Email Updates
Find out about opportunities to join the movement, the latest breakthroughs and how they can help you live your best life with MS.
Health Conditions
- Alzheimer's & Dementia
- Asthma & Allergies
- Atopic Dermatitis
- Breast Cancer
- Cardiovascular Health
- Environment & Sustainability
- Exercise & Fitness
- Headache & Migraine
- Health Equity
- HIV & AIDS
- Human Biology
- Men's Health
- Mental Health
- Multiple Sclerosis (MS)
- Parkinson's Disease
- Psoriatic Arthritis
- Sexual Health
- Ulcerative Colitis
- Women's Health
Health Products
- Nutrition & Fitness
- Vitamins & Supplements
- At-Home Testing
- Men’s Health
- Women’s Health
- Latest News
Original Series
- Medical Myths
- Honest Nutrition
- Through My Eyes
- New Normal Health
- 5 things everyone should know about menopause
- 3 ways to slow down type 2 diabetes-related brain aging
- Toxic metals in tampons: Should you be worried?
- Can tattoos cause blood or skin cancer?
- Can we really ‘outrun the Grim Reaper’?
- What makes a diet truly heart-healthy?
General Health
- Health Hubs
Health Tools
- Find a Doctor
- BMI Calculators and Charts
- Blood Pressure Chart: Ranges and Guide
- Breast Cancer: Self-Examination Guide
- Sleep Calculator
- RA Myths vs Facts
- Type 2 Diabetes: Managing Blood Sugar
- Ankylosing Spondylitis Pain: Fact or Fiction
About Medical News Today
- Our Editorial Process
- Content Integrity
- Conscious Language
Find Community
- Bezzy Breast Cancer
- Bezzy Psoriasis
Multiple sclerosis research: Where are we now?

Multiple sclerosis (MS) causes a wide range of symptoms involving the brain, optic nerves, and spinal cord. Research is only just beginning to reveal who is at risk and what causes the condition.

MS is a chronic condition affecting 2.8 million people worldwide. While treatment options are currently limited, trials of several new approaches are underway.
Researchers believe that MS is an autoimmune disorder. This type of illness involves the immune system attacking healthy cells, much as it would attack viruses or bacteria.
In the case of MS, the immune system attacks the myelin sheath that surrounds nerve cells. The attack causes lesions to form, and over time, these cause scarring, which leads to nerve damage and reduced function.
As a result of this damage, a person with MS may experience numbness and tingling sensations, fatigue , muscle weakness, dizziness and vertigo , memory issues, and vision problems, among other symptoms .
There are four types of MS: clinically isolated syndrome (CIS), relapsing-remitting MS, primary progressive MS, and secondary progressive MS.
CIS is a single episode of MS-like symptoms that lasts for at least 24 hours. People with CIS do not necessarily have MS, but experiencing an episode can be the first sign of the condition.
Treating MS involves interdisciplinary care, including rehabilitation, disease-modifying drugs (DMARDs), and complementary and alternative therapies .
Scientists do not fully understand the risk factors for MS and the mechanisms of the condition. However, they are making new headway in the search for answers and improvements in treatment.
What does the latest research show about the risk factors, mechanisms, and treatments of MS? In this Special Feature, Medical News Today takes a closer look.
A brief look at the history of MS
French neurologist Jean-Martin Charcot first described the features of MS in 1868 . He noted the differences between this condition and the tremor of paralysis agitans, a symptom of the neurological condition later named Parkinson’s disease.
The three symptoms associated with MS at the time were called Charcot’s triad. They included a characteristic tremor, involuntary eye movements, also known as nystagmus , and scanning speech , which some call staccato or explosive speech.
Decades later, the invention of MRI scans helped doctors diagnose MS. Treatment with steroids became commonplace, and doctors then began to use medications in a class of drugs called interferons. The Food and Drug Administration (FDA) first approved interferons for use in people with MS in 1993 .
Article highlights:
The unknowns of MS
Although scientists and healthcare professionals understand the defining features of MS, several aspects of the condition remain a mystery.
While researchers recognize that MS is an autoimmune condition, they do not understand why immune cells attack myelin.
Also, diagnosing MS is still an ambiguous process because its symptoms are similar to those of many other health conditions.
In addition, experts do not know why women are 2–3 times more likely to be diagnosed with MS than men.
Who is at risk of MS?
Research suggests that risk factors of MS include a lack of vitamin D or sunlight, smoking, obesity , a history of infection with the Epstein-Barr virus, being female, and possibly having inherited specific genes, as well as environmental factors.
More recently, the gut microbiota has emerged as a possible risk modulator.
A recent overview of clinical research found that people with MS had larger populations of Pedobacteria , Flavobacterium , Pseudomonas , Mycoplana , Acinetobacter , Eggerthella , Dorea , Blautia , Streptococcus , and Akkermansia bacteria in their intestines than people without MS.
People with MS also had reduced populations of Prevotella , Bacteroides , Parabacteroides , Haemophilus , Sutterella , Adlercreutzia , Coprobacillus , Lactobacillus , Clostridium , Anaerostipes , and Faecalibacterium bacteria.
Researchers speculate that balancing out the populations of gut bacteria in people with MS may reduce inflammation and the overactivation of the immune system.
How do MS mechanisms affect nerve cells?
Research from the MS Society Edinburgh Centre for MS Research found that people with MS had reduced numbers of inhibitory neurons , compared with people who did not have the condition.
However, people with MS had as many stimulating neurons as those without the condition. This was true even for people who had received their MS diagnoses many years earlier.
These findings help reveal the types of neurons affected by MS, shedding more light on how the condition evolves within the body. The research may also offer insight into treatments that could protect the targeted neurons.
What are the latest treatments for MS?
DMARDs that health authorities have recently approved as MS treatments include cladribine ( Mavenclad ) and siponimod ( Mayzent ) for relapsing-remitting and active secondary progressive forms of the condition.
Cladribine targets lymphocytes, white blood cells responsible for attacks on myelin. Siponimod harnesses specific white blood cells that attack myelin and prevents them from circulating in the body.
However, due to their interactions with the immune system, these drugs may lead to a reduction in lymphocytes, making a person vulnerable to infections.
The medicines’ actions may also contribute to reduced responses to vaccines in people who receive routine vaccinations. With the introduction of COVID-19 vaccines, scientists have investigated whether people with MS who take medications such as cladribine can have adequate responses to vaccines.
The latest research indicates that people taking cladribine do produce protective antibodies to other common vaccines, despite having decreased lymphocyte levels induced by the medication.
This result gives scientists and others in the medical community hope that people who take these drugs for MS will have similarly adequate responses to COVID-19 vaccines.
Research on the horizon
Some scientists are currently investigating the potential for stem cell therapy for MS. In a phase 1 study conducted at the Karolinska Institute, in Stockholm, Sweden, seven people with progressive MS received infusions of stem cells derived from each participant’s own bone marrow .
As early as 7 days after administration of the stem cell therapy, researchers found evidence of positive changes in the participants’ immune systems. At 12 weeks, five out of six participants had no new characteristic lesions on follow-up MRI brain scans.
As their understanding of the condition evolves, many scientists are investigating the root cause of MS.
An analysis of the current data has revealed a possible connection between gut health and the condition. Data revealing relationships between the gut microbiota and the brain continually emerge, and scientists are hopeful that diet modifications, probiotics, and certain drugs that balance the gut microbiome will play a role in MS treatment.
Also in development are remyelination and neuroprotection therapies. The latter aim to protect the axons and myelin from further damage, while the former could restore lost function for people with MS.
Meanwhile, immunotherapy drugs would protect the nerves from destruction and rebuild neurons that have already sustained damage.
Another potential treatment in phase 1 trials is a tumor necrosis factor-alpha (TNF-alpha) inhibitor called MYMD-1 . TNF-alpha is a type of cytokine produced by white blood cells that regulates some aspects of the immune system.
Overproduction of this cytokine is associated with several autoimmune conditions, including MS. MYMD-1 is a new type of TNF-alpha blocker that shows promise as a treatment for MS and other conditions.
Trials for therapies involving the gut microbiome , stem cells , neuroprotective treatments , remyelination , and MYMD-1 are still in the earliest stages. However, the possibilities provide hope that ongoing research will lead to effective ways to prevent MS and better methods of treatment.
- Multiple Sclerosis
- Immune System / Vaccines
- Neurology / Neuroscience
Share this article
Latest news
- 3 ways to boost longevity in 2025
- Antibiotic use does not increase dementia risk, study suggests
- Insufficient sleep and high blood pressure may raise risk of brain aging
- Why getting more deep sleep may help improve memory
- Low carb diet may improve cholesterol levels in people with type 2 diabetes
Related Coverage
What is the most recent research on multiple sclerosis, and are there any new treatments on the horizon? We spoke to three experts to find out more.
MNT speaks to Dr. Marianna Cortese, a multiple sclerosis expert, and Dr. Antje Ronneberger, who lives with MS, about the latest research findings.
Understanding multiple sclerosis, its symptoms, and how it can progress helps people to assess themselves for this condition. Learn more about MS here.
Multiple sclerosis is an autoimmune disease, the causes of which remain mysterious. But a new study may now have uncovered a key factor in its…
Gilenya is a prescription drug used to treat multiple sclerosis. Learn how to lower long-term costs and more.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Current and emerging therapies in multiple sclerosis: a systematic review
Wanda castro-borrero, donna graves, teresa c frohman, angela bates flores, paula hardeman, diana logan, megan orchard, benjamin greenberg, elliot m frohman.
- Author information
- Article notes
- Copyright and License information
Email: [email protected]
Corresponding author.
Issue date 2012 Jul.
Multiple sclerosis (MS) is a potentially disabling chronic autoimmune neurological disease that mainly affects young adults. Our understanding of the pathophysiology of MS has significantly advanced in the past quarter of a century. This has led to the development of many disease-modifying therapies (DMTs) that prevent exacerbations and new lesions in patients with relapsing remitting MS (RRMS). So far there is no drug available that can completely halt the neurodegenerative changes associated with the disease. It is the purpose of this review to provide concise information regarding mechanism of action, indications, side effects and safety of Food and Drug Administration and European Medicines Agency approved agents for MS, emerging therapies, and drugs that can be considered for off-label use in MS.
Keywords: disease-modifying therapies, emerging therapies, fingolimod, glatiramer acetate, interferon β, multiple sclerosis, natalizumab
Introduction
Multiple sclerosis (MS) is a chronic autoimmune inflammatory disease of the central nervous system (CNS) that mainly affects young adults and may lead to significant disability over time. Since the first documented case of MS in the nineteenth century the knowledge regarding the pathophysiology of the disease has significantly advanced. The inflammatory cells in MS have been well described and include CD4 and CD8 T lymphocytes, microglia and macrophages [ Goverman, 2011 ]. Also humoral immunity has been described as an important component in the pathophysiology of MS [ Boster et al. 2010 ].
Within the past 30 years new and effective therapies have been developed that decreased clinical relapses, reduced new T2 and gadolinium-enhancing (Gad+) lesions and aim to halt the progression of disease. Since the US Food and Drug Administration (FDA) approval of the first disease-modifying therapy (DMT) in 1993, interferon (IFN)-β1b (Betaseron), which was also approved in Europe in 1995 under the name of Betaferon, we now have a total of eight FDA-approved therapies for MS, including an oral agent and a single agent approved for secondary progressive MS (SPMS) ( Table 1 ). Of note, there are two agents approved by the European Medicines Agency (EMA) for the treatment of SPMS, mitoxantrone and IFN-β1b (Betaferon/Extavia). All first-line injectable agents have been studied in clinically isolated syndrome (CIS) and have demonstrated decreased risk of conversion into clinically definite MS (CDMS) ( Table 2 ) [ Kappos et al. 2006 ; Jacobs et al. 2000 ; Comi et al. 2001 , 2009 , 2012a ]. So far there is no effective therapy to halt progression of disease and reduce disability in primary progressive MS (PPMS).
Current Food and Drug Administration/European Medicines Agency approved therapies for multiple sclerosis (MS)
AV, atrioventricular; CBC, complete blood count; IFN, interferon; LFT, liver function test; LVEF, left ventricular ejection fraction; PML, progressive multifocal leukoencephalopathy; PF, pulmonary function; TSH, thyroid-stimulating hormone; U/A, urinalysis; UTI, urinary tract infections.
Selection of side effects, not full side effects profile.
Pivotal trials for approval of disease-modifying therapies in clinically isolated syndrome
BENEFIT, Betaseron/Betaferon in newly emerging multiple sclerosis for initial treatment; CHAMPS, the controlled high risk Avonex multiple sclerosis trial; ETOMS, early treatment of multiple sclerosis; IFN, interferon; PreCISe, effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome; REFLEX, REbif FLEXible dosing in early MS.
There are many new agents in the pipeline which will bring great choices into the MS pharmacological armamentarium ( Table 3 ).
Multiple sclerosis emerging therapies.
ARR, annualized relapse rate; DEFINE, efficacy and safety of oral BG00012 in relapsing remitting multiple sclerosis; HZV, Herpes Zoster virus; LFT, liver function test; Th, T helper; UTI, urinary tract infection.
FDA- and EMA-approved therapies
Interferon β.
IFNs are a family of proteins that play a role in the body’s natural defense against microbial, neoplastic and viral insults and have a role in regulating the immune response. IFN-β impacts the immune system in several ways, such as decreasing major histocompatibility complex (MHC) class II expression, upregulation of interleukin 10 (IL-10) production, and decreased T helper (Th)-1 and Th17 production, which leads to an overall anti-inflammatory effect [ Kieseier, 2011 ; Kappos et al. 2007 ].
Subcutaneous interferon β1b (Betaseron, Bayer Schering Pharma AG/Betaferon, Bayer Schering Pharma AG/Extavia, Novartis Pharmaceuticals Corp.)
The pivotal phase III trial using IFN-β1b was a randomized, double-blind, placebo-controlled, multicenter trial of 372 patients with RRMS over 2 years. This trial demonstrated a 34% reduction in overall relapses compared with placebo. More specifically, there was a 50% reduction in annualized relapses classified as moderate to severe in the treatment group. Patients receiving IFN-β1b were also found to have a lower T2 lesion volume and decreased accumulation of new lesions [ IFNB Multiple Sclerosis Study Group. 1993 ]. Each of the IFN-β therapies, as well as glatiramer acetate, has been shown to delay conversion to CDMS in patients with CIS ( Table 2 ). In the 5-year active treatment extension of the BENEFIT trial, the effects of early versus delayed treatment with IFN β1b were investigated. This study showed the risk of conversion to CDMS remained lower in the group receiving early treatment; 46% compared with 57% of patients converting from CIS to CDMS [hazard ratio (HR) 0.63; 95% confidence interval (CI) 0.48–0.83; log rank test p = 0.003) [ Kappos et al. 2009 ].
Intramuscular interferon β1a (Avonex, Biogen Idec, Inc.)
In the pivotal trial including 301 patients with RRMS, IFN-β1a intramuscularly was shown to delay time to progression of disability with fewer treated subjects experiencing disability progression (21.9% versus 34.9%; p = 0.02) compared with placebo. Annualized relapse rates (ARRs) over a 2-year period were also lower compared with placebo (ARR 0.61 versus 0.90; p = 0.03). The accumulation of Gad+ lesions was also reduced; however, T2 lesion volume was not significantly different between the two groups at 2 years [ Jacobs et al. 1996 ].
Subcutaneous interferon β1a (Rebif, EMD Serono, Inc.)
The Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis (PRISMS) trial was a 2-year randomized, double-blind, placebo-controlled, multi-centered trial of 560 patients with RRMS. Subjects treated with either the 22 or 44 μg dose of IFN-β1a subcutaneously showed a significant reduction in ARRs compared with placebo, 27% and 33% respectively. Both treatment groups showed a significant reduction in the number of new or enlarging T2 lesions; 67% reduction in the 22 μg group and 78% reduction in the 44 μg group [ PRISMS Study Group, 1998 ]. An extension study utilizing a crossover design in which placebo-treated patients were randomized to either 22 or 44 μg of IFN-β1a subcutaneously after 2 years revealed patients in both active treatment groups for the entire 4 years continued to show significantly lower number of relapses per year [PRISMS Study Group, 2001]. IFNs have immunogenic properties and treated individuals may develop binding and neutralizing antibodies (NAbs) to these products. NAbs may develop with the use of all formulations of IFN-β; however, they are found more commonly with the high-dose, high-frequency IFNs (IFN-β1b and IFN-β1a subcutaneously). The issue of NAbs is controversial; however, a panel of experts met at the Neutralizing Antibodies on Interferon Beta in Multiple Sclerosis (NABINMS) consortium in 2009 in attempts to formulate a practical approach to the evaluation and incorporation of information regarding NAbs in the treatment of MS. The group proposed that both the NAb titer and clinical status of the patient should be considered in the decision regarding the impact of the presence of NAbs on changing DMTs. They also suggested reevaluation of the NAbs status prior to making a change in therapy unless patients were clearly performing poorly clinically [ Polman et al. 2010 ].
Glatiramer acetate
Glatiramer acetate (GA) (Copaxone, Teva Neuroscience North America / Teva Pharmaceuticals) is a first-line therapy for relapsing forms of MS and CIS. GA contains an incalculable number of active amino acid sequences and is composed of a large number of synthetic peptides. The usual dose of GA is 20 mg subcutaneously once a day.
The mechanism of action (MOA) of GA is not completely understood, but consists of an antigen-presenting cell (APC) incorporating peptides of GA and presenting them to a lymphocyte, similar to the process of a vaccine. This process creates a unique population of lymphocytes circulating in the blood which are responsive to GA. It inhibits the multiplication of human lymphocytes that are capable of reacting to myelin basic protein. Researchers have been able to show that GA binds directly to the portion of the APC that is required to stimulate the T lymphocyte, thus blocking direct immunologic attack. It was in the late 1980s that the immunologic concept of Th1 (proinflammatory) and Th2 (anti-inflammatory) lymphocytes gained momentum. These two types of lymphocytes can be identified by the chemicals that they manufacture and then secrete. These chemicals are known as cytokines, and can be divided into inflammatory and proinflammatory. In 1997, Aharoni and colleagues published a paper that described how GA could stimulate the production of Th2 (anti-inflammatory) cells that inhibited the inflammatory response by secreting anti-inflammatory cytokines [ Aharoni et al. 1997 ]. The GAs’ effect begins in the peripheral tissues in a population of specific lymphocytes which circulate in the blood and are capable of migrating into the CNS tissue by crossing the blood–brain barrier (BBB). These cells then encounter fragments of several myelin proteins that stimulate the glatiramer cells to multiply and begin to produce anti-inflammatory cytokines. Since the glatiramer-activated lymphocytes can suppress inflammation under way in the diseased area of CNS tissue, this process has been given the name bystander suppression [ Johnson, 2010 ]. To date, data suggest that GA treatment is associated with a broader immunomodulatory effect on cells of not only the innate but also the adaptive immune system. Recent investigations indicate that GA treatment may also promote regulatory B-cell properties [ Lalive et al. 2011 ].
GA has a relatively narrow adverse effect profile. Most frequently patients complain of mild pain and pruritis at the injection site. Lipoatrophy and skin site reactions are also seen and may lead to discontinuation of therapy. A transient reaction called immediate postinjection reaction consists of chest tightness, flushing and dyspnea beginning soon after the injection and lasting no longer than 20 min. If no history or evidence of coronary artery disease, the patient can be reassured that such a reaction is benign [ DiPiro et al. 2005 ].
Multicenter trials with GA have demonstrated statistically significant reductions in mean ARR that are comparable to those of the IFNs [ DiPiro et al. 2005 ]. In two recent studies the efficacy of GA was compared with high-dose/high-frequency IFN-β. In the Rebif versus Glatiramer Acetate in Relapsing MS Disease (REGARD) study [ Mikol et al. 2008 ], subcutaneous IFN-β1a was compared with GA, and in the Betaseron/Betaferon Efficacy Yielding Outcomes of a New Dose (BEYOND) study [ O’Connor et al. 2009 ], subcutaneous IFN-β1b was compared with GA. In both trials, there was no significant difference between IFN and GA in the primary endpoints or in any clinical endpoints, although some differences in magnetic resonance imaging (MRI) measures of disease activity have been claimed.
The results from a 15-year analysis of the US prospective open-label study of GA indicate that long-term continuous use is safe. It also indicates that the majority of patients continuing on GA therapy in the study have had few relapses and minimal disease progression. Of the initial 232 patients that received at least one GA dose since study initiation in 1991, only 100 (43%, ongoing cohort) patients continued. Of the 100 patients receiving continuous GA as sole immunomodulatory therapy for 15 years (mean disease duration of 22 years and mean patient age of 50 years) have not transitioned to SPMS, 57% have retained stable or improved the Expanded Disability Status Scale (EDSS) scores over the course of the study and 82% remain ambulatory without mobility aids. There was no occurrence of any unforeseen adverse events in patients receiving GA therapy. The study will continue for 20 years of prospective follow up [ Ford et al. 2010 ].
Mitoxantrone
Mitoxantrone is an anthracenedione initially developed as an anti-neoplastic agent that reduces lymphocyte proliferation. Mitoxantrone intercalates into DNA strands, inducing strand breakage and inhibition of the DNA repair enzyme topoisomerase II. It is an immunosuppressive agent used as a second-line treatment for SPMS, primary relapsing multiple sclerosis and worsening RRMS. Mitoxantrone was approved for the treatment of SPMS based on the study by Hartung and colleagues [ Hartung et al. 2002 ].
Several studies have shown it to be efficacious in reducing exacerbations and number of Gad+ lesions on MRI, and it seems to have effects on disease course up to 5 years after discontinuing therapy [ Martinelli et al. 2009 ; Goodin et al. 2003 ]. Mitoxantrone is given as an intravenous infusion over 30 min every 3 months at 12 mg/m 2 for a 2- to 3-year period with a maximum cumulative dose of 140 mg/m 2 . Common side effects include alopecia, nausea and vomiting, an increased risk of infection (particularly urinary and respiratory tracts infections) and amenorrhea. Mitoxantrone, though effective, remains second line due to its risk of two serious adverse effects that can occur at any time after the first dose is given. The first, acute leukemia has an incidence of approximately 0.81% [ Marriott et al. 2010 ]. Regular monitoring of complete blood counts is recommended. Mitoxantrone can also cause decreased left ventricular ejection fraction (LVEF) and congestive heart failure at a rate of approximately 12% and 0.4%, respectively [ Marriott et al. 2010 ]. To monitor cardiotoxicity, a baseline LVEF must be obtained and any patient with an ejection fraction less than 50% should not receive mitoxantrone. It was previously believed that cardiotoxicity could only occur with cumulative doses over 96–140 mg/m 2 ; however, several reports of cardiotoxicity below this threshold have caused the FDA to recommend monitoring cardiac function before every infusion. The therapy must be discontinued if the LVEF ever falls below 50% or decreases by 10% [ Martinelli et al. 2009 ].
Natalizumab
Migration of leukocytes from the vasculature into the parenchyma involves the interaction between leukocyte adhesion molecules and their complementary ligands on vascular endothelial cells. Leukocyte integrins are heterodimeric glycoproteins that contain an α and β chain [ Ransohoff, 2007 ]. Vascular cell adhesion molecule 1 (VCAM-1) is expressed on the surface of vascular endothelial cells in the blood vessels within the CNS and interacts with α4β1 integrin on lymphocytes to allow for extravasation across the BBB. Also, the interaction of α4β1 integrin with fibronectin and osteopontin may modulate the survival, priming and activation of leukocytes that have entered into the parenchyma of the brain and spinal cord. Natalizumab (Tysabri, Biogen Idec, Inc.) contains humanized immunoglobulin G4κ monoclonal antibodies against leukocyte α4 integrins, including α4β1 and α4β7 integrins, and blocks binding to their endothelial receptors (VCAM-1 and mucosal addressin cell adhesion molecule 1, respectively) [ Polman et al. 2006 ]. By blocking α4 integrins, natalizumab inhibits the migration of leukocytes into the brain, which results in reduced inflammation.
Natalizumab was evaluated for the treatment of RRMS in two phase III clinical trials. The Natalizumab Safety and Efficacy in relapsing remitting multiple sclerosis (AFFIRM) study evaluated 942 patients who were randomly assigned to receive natalizumab versus placebo every 4 weeks for 2 years. The primary endpoints were the rate of clinical relapse at 1 year and the rate of sustained progression of disability, measured by the EDSS, at 2 years. Natalizumab reduced the risk of sustained disability by 42% over 2 years (HR 0.58; 95% CI 0.43–0.77; p < 0.001). It reduced the rate of clinical relapse at 1 year by 68% ( p < 0.001). MRI scans were obtained at baseline, 1 year and 2 years. Treatment with natalizumab resulted in an 83% reduction of new or enlarging hyperintense T2 lesions over 2 years (mean number of lesions 1.9 with natalizumab and 11 with placebo; p < 0.001). There were 92% fewer Gad+ lesions in the natalizumab group than in the placebo group at 1 and 2 years ( p < 0.001). There was also a significant effect on Gad+ lesions seen after 6 weeks of natalizumab treatment [ Polman et al. 2006 ].
The Safety and Efficacy of Natalizumab in combination with IFN-β1a in patients with RRMS (SENTINEL) trial was a 2-year phase III trial evaluating treatment with natalizumab or placebo in combination with IFN-β1a. The primary endpoints were the rate of clinical relapse at 1 year and accumulative probability of disability progression, measured by the EDSS, at 2 years. The study showed that treatment with both drugs was more effective than treatment with IFN-β1a alone. Patients on combination treatment were less likely to have sustained disability progression (23% versus 29%) and were more likely to remain relapse free (61% versus 37%). Combination treatment resulted in fewer new or enlarging T2 lesions (0.9 versus 5.4; p < 0.001) [ Rudick et al. 2006 ]. The study ended a month early due to the occurrence of progressive multifocal leukoencephalopathy (PML) in two patients who received natalizumab with IFN-β1a.
The most notable potential adverse effect of natalizumab treatment is the development of PML. Following the observation that three patients treated with natalizumab developed PML, it was withdrawn from the market in February 2005 and reintroduced in July 2006 as monotherapy treatment for RRMS. The original risk of PML was estimated to be approximately one per 1000 patients receiving natalizumab [ Berger, 2010 ]. As of 4 January 2012, approximately 96,582 patients have received natalizumab since it was marketed and there have been 201 confirmed cases of PML worldwide. Approximately 20% of patients who have developed PML have died. Those that survived have varying levels of disability, ranging from mild to severe. Fewer patients treated and wide confidence intervals result in questionable estimates beyond 30 months of treatment.
PML is a rare demyelinating disease of the brain due to the John Cunningham (JC) virus. It is almost always seen in association with an underlying immunosuppressive condition. The precise explanation for the increased risk of PML with natalizumab therapy remains unknown.
In the natalizumab clinical trials, there was a small increase in the rate of infections, including herpes infections, pneumonia and urinary tract infections. There were no other opportunistic infections or increase cases of cancer reported [ Ransohoff, 2007 ]. Post-release monitoring disclosed one case of fatal herpes encephalitis, one nonfatal case of herpes meningitis, cryptosporidium gastroenteritis, pneumocystis carinii pneumonia, varicella pneumonia and mycobacterium avium intracellular complex pneumonia [ Ransohoff, 2007 ; Gorelik et al. 2010 ].
Natalizumab infusions were complicated by serious hypersensitivity reactions, including fever, rash and anaphylaxis, in less than 1% of patients and less serious infusion reactions in about 4% of patients [ Ransohoff, 2007 ; Polman et al. 2006 ; Rudick et al. 2006 ]. Patients with infusion reactions were more likely to have persistent NAbs. The presence of antibodies lessoned natalizumab’s clinical efficacy and resulted in clinical and radiographic disease activity equivalent to patients in the placebo group [ Ransohoff, 2007 ].
Natalizumab is an extremely effective therapy for RRMS and is licensed for highly active naïve patients. Due to the potential risk of PML and other opportunistic infections, it is typically reserved for patients with clinically or radiographically extremely active disease either as initial therapy or when initial therapy has been ineffective or poorly tolerated. Treatment with natalizumab requires rigorous ongoing clinical surveillance. To minimize the risk of PML, patients beginning treatment should have no history of immunosuppressive medications in the preceding 3 months and should not have other conditions that may compromise cell-mediated immunity. The FDA and EMA recommend the use of the JC virus antibody for risk stratification on all patients on Tysabri. The risk of PML increases after 24 months on therapy, if there has been prior immunosuppressant use and the presence of JC virus antibody. Patients with positive JC virus antibody, prior treatment with an immunosuppressant and who have received more than 24 doses of Tysabri have an estimated risk of PML of 9–11/1000. However, patients without any of those risk factors for PML have a risk of PML of less than 0.1 per 1000 [ Sorensen et al. 2012 ].
Fingolimod is an oral sphingosine-1 phosphate (S1P) receptor modulator. It was approved by the FDA in September 2010 as first-line therapy for RRMS. However, the EMA has recommended that its use be limited to those whose condition fails to respond to first-line therapy or only in cases of severe, rapidly developing cases of MS. It acts as a sphingosine analogue, binding to the S1P 1 receptor on lymphocytes leading to internalization and downregulation of their expression and thereby preventing the egression of lymphocytes from the lymph nodes. Additionally, through interactions with S1P receptors on neural cells, fingolimod has been shown to have potentially neuroprotective effects in the animal experimental autoimmune encephalomyelitis model [ Foster et al. 2007 ; Coelho et al. 2007 ; Miron et al. 2008 ].
In the 24-month phase III FTY720 Research Evaluating Effects of Daily Oral therapy in Multiple Sclerosis (FREEDOMS) trial comparing placebo with oral fingolimod at doses of 1.25 mg and the now FDA-approved 0.5 mg daily dose, there was a significant reduction in ARR with both doses of fingolimod (0.16 at 1.25 mg and 0.18 at 0.5 mg) compared with placebo (0.40) which represented a relative reduction of 60% and 54%, respectively. Furthermore, fingolimod also reduced the risk of disability progression with a probability of disability progression (confirmed after 3 months) of 17.7% at the 0.5 mg dose and 16.6% at the 1.25 mg dose compared with 24.1% with placebo. Almost 90% of patients receiving fingolimod, at either dose, were free of enhancing lesions over the course of 2 years and approximately 50% were free of new or enlarging T2 lesions [ Kappos et al. 2010 ].
The Trial Assessing Injectable Interferon versus FTY720 Oral in RRMS (TRANSFORMS) comparing fingolimod with intramuscular INF-β1a showed a 52% relative reduction in ARR in the patients treated with fingolimod 0.5 mg versus IFN. This study showed a similar beneficial effect on MRI markers compared with IFN-β1a; however, there was no statistically significant difference in the disability progression between the fingolimod and IFN-β1a groups [ Cohen et al. 2010 ].
Despite its efficacy, there are additional safety concerns compared with the injectable therapies. Data from the two pivotal trials showed an increased risk of infections, cardiovascular effects, including bradycardia and atrioventricular (AV) block (first and second degree) with initial dosing and macular edema. Each of these was more common with the higher 1.25 mg dose. Of note, there were two deaths related to infections in subjects receiving fingolimod at the 1.25 mg dose in TRANSFORMS. One death was secondary to a dissemination varicella zoster infection and the second was related to herpes simplex encephalitis. While herpes virus infection has been seen at the 0.5 mg dose, cases tended to be mild and were not found to occur at a higher rate than the control arm [ Cohen et al. 2010 ].
The EMA recently recommended increased patient monitoring during the first dose of fingolimod, including electrocardiogram monitoring before treatment and then continuously for the first 6 h after the first dose is administered, and measurement of blood pressure and heart rate every hour over the same 6 h.
Off-label therapies
Immunosuppressive agents, chemotherapies and various mAbs have been used off label for many years as DMTs in MS but the potential benefits of these therapies are limited by systemic adverse events, such as increased risk of malignancy and opportunistic infections. These agents have been used in patients who are refractory to or cannot tolerate the side effects of IFN-β and GA, cannot afford FDA-approved therapies, or need intensification of therapy (i.e. used in combination with IFN-β or GA). Also limiting the use of these medications is the lack of large-scale, controlled trials, validating their efficacy.
Mycophenolate mofetil
Mycophenolate mofetil (MMF; Cellcept, Roche Laboratories, Nutley, NJ, USA) is FDA and EMA approved for preventing rejection of cardiac, liver and renal transplants. MMF undergoes rapid and complete metabolism to mycophenolic acid (MA), which is the active metabolite. MA is a potent, selective, noncompetitive and reversible inhibitor of inosine 5’ monophosphate dehydrogenase type II. MA inhibits the de novo synthesis pathway of guanosine nucleotides without being incorporated into DNA. Because T and B lymphocytes are critically dependent for their proliferation on de novo synthesis of purines, while other cell types can utilize salvage pathways, MA has potent cytostatic effects on lymphocytes. MA inhibits proliferative responses of T and B lymphocytes to both mitogenic and allospecific stimulation. MA also suppresses antibody formation by B lymphocytes [ Product information: Cellcept, 2009 ].
Potential side effects include hypertension, backache, abdominal pain, diarrhea, nausea, elevated transaminases, vomiting, anxiety and tremor. Serious side effects include gastrointestinal bleeding, thrombocytopenia, skin cancer, opportunistic infection and PML. Increased susceptibility to infection and the possible development of lymphoma may result from immunosuppression.
A retrospective review of experience in treating 79 patients with MS with MMF showed that this agent was well tolerated by the majority of patients. Patients were initiated on 500 mg twice a day, which was titrated up by 500 mg weekly to a maximum of 1000 mg twice a day. While the observations were uncontrolled, some of the patients demonstrated either stabilization or improvements in their activities of daily living, ambulation and relapse rate [ Frohman et al. 2004 ]. In a randomized, MRI-blinded, parallel group, pilot trial of MMF compared with IFN-β1a, both drugs appeared safe and well tolerated in the majority of patients. The trial also showed a trend toward a lower accumulation of combined active MRI lesions. MMF showed a nonstatistically significant increase in infections [ Frohman et al. 2010 ]. The dose generally used in patients with RRMS is 1000 mg twice daily. Large, randomized clinical trials are needed to better evaluate the safety and efficacy of this agent in patients with MS.
Azathioprine
Azathioprine is FDA approved for rejection prophylaxis (as monotherapy or adjunct) of renal transplant and rheumatoid arthritis (RA). Although not FDA approved, it has been used in the USA to treat MS since 1971 [ La Mantia et al. 2007 ]. Azathioprine is licensed for MS therapy in Germany. Azathioprine is an imidazole derivative of 6-mercaptopurine and acts as an immunosuppressive antimetabolite. It is a purine antagonist and affects DNA replication. It impairs T-cell lymphocyte function and is more selective for T lymphocytes than for B lymphocytes [ Casetta et al. 2009 ]. The Cochrane MS Group concluded that azathioprine is an appropriate maintenance treatment for patients with MS and could be a fair alternative to IFN. It is recommended that cumulative doses do not exceed 600 g due to possibly increasing the risk of malignancies [ Casetta et al. 2009 ].
Methotrexate
Methotrexate (MTX) is a chemotherapeutic agent used for the treatment of severe psoriasis, juvenile RA (JRA), severe RA, acute lymphoid leukemia and other malignancies. MTX reversibly inhibits dihydrofolate reductase. Via this mechanism, MTX sodium interferes with DNA synthesis, repair and cellular replication [ Product information: methotrexate, 2000 , 2005 ].
On a systematic review of oral MTX for MS, for the Cochrane Multiple Sclerosis Group, the authors do not recommend the use of MTX for progressive MS or RRMS due to a lack of high-quality evidence. Future trials need to be performed using standard outcome measures and objective measures, such as MRI [ Gray et al. 2004 ].
Rituximab is FDA approved for the treatment of non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, refractory moderate to severe RA, Wegener’s granulomatosis and microscopic polyangiitis [ Prescribing information, 2010 ]. It is EMA approved for diffuse large B-cell lymphoma and autoimmune arthritis. Rituximab is a chimeric murine/human mAb that targets and selectively binds CD20, an antigen present on pre-B cells and B cells, but not on antibody-producing plasma cells or stem cells in the bone marrow. By binding CD20, rituximab depletes circulating B-cell populations (but not stem cells or plasma cells) through a combination of cell-mediated and complement-dependent cytotoxicity and possibly promoting apoptosis [ Bar-Or et al. 2008 ].
Common side effects of rituximab include nasopharyngits, urinary tract infections, nausea, leucopenia, fever, fatigue, headache, muscle spasms and diarrhea. Cases of PML, severe mucocutaneous reactions, tumor lysis syndrome and fatal infusion reactions have been documented. Other severe adverse reactions include fulminant hepatitis, hepatic failure, bacterial, fungal or viral infections, cardiac arrhythmias, renal toxicity and bowel obstruction or perforation [ Prescribing information, 2010 ].
In a 72-week, open-label phase I trial the safety and tolerability of rituximab were evaluated in 26 patients with RRMS. The authors indicated that no serious adverse events were reported in this small cohort with active RRMS and all the adverse events including infections were mild to moderate and did not lead to medication withdrawal. No efficacy conclusions were noted due to the absence of a control group but they noticed a reduction in relapses, Gad+ lesions, new T2 lesion number and T2 lesion volumes through 72 weeks [ Bar-Or et al. 2008 ]. In a phase II randomized, placebo-controlled trial with 104 patients there was a reduction in Gad+ lesions and relapses in patients on rituximab versus placebo [ Hauser et al. 2008 ]. Rituximab has shown efficacy in the treatment of patients with RRMS. A recently completed randomized clinical trial using a standard dose of rituximab in patients with RRMS demonstrated a 91% reduction in the number of Gad+ lesions on MRI, as well as a significant reduction in the number of clinical relapses [ Hauser et al. 2008 ]. In a recent trial of patients with PPMS, rituximab appeared to have efficacy only in young patients (primarily male) with signs of active inflammation on MRI scans [ Hawker et al. 2009 ]. Manufacturers of rituximab decided not to go forward with phase III trials, but other CD20 molecule manufacturers are undertaking phase III trials.
Immunoglobulin
While several studies have suggested a beneficial effect of intravenous immunoglobulin (IVIG) in RRMS [ Fazekas et al. 1997 ; Achiron et al. 1998 ; Lewanska et al. 2002 ] and CIS [ Achiron et al. 2004 ] in terms of relapse rate, MRI and disability progression, there were limitations in terms of methodology and sample size. The most recent published trial from the Prevention of Relapse with Intravenous Immunoglobulin (PRIVIG) study group brought the efficacy of IVIG as a preventative agent in MS into question [ Fazekas et al. 2008 ]. Current guidelines from the European Federation of Neurological Societies recommend that IVIG be considered as a second- or third-line therapy in RRMS if conventional immunomodulatory therapies are not tolerated [ Elovaara et al. 2008 ]. However, IVIG is widely used to reduce relapse rate following pregnancy [ Achiron et al. 1996 ; Haas and Hommes, 2007 ].
Corticosteroids
The use of corticosteroids in MS has primarily focused on treatment of exacerbations, but there is evidence to support the use of steroids as a preventative therapy. Data from the Optic Neuritis Treatment Trial (ONTT) suggest that the use of high-dose steroids reduces the risk of development of MS at 2 years following the initial optic neuritis event [ Beck et al. 1993 ]. Further, a randomized, controlled phase II trial utilizing pulsed high-dose methylprednisolone over the course of 5 years showed a reduction in brain atrophy, T1 lesion volume and disability progression, but failed to show a difference in annualized relapse rate or T2 lesion volume [ Zivadinov et al. 2001 ].
Methylprednisolone in combination with IFN-β1a did not show reduction in disability progression compared with IFN-β1a alone [ Ravnborg et al. for The MECOMBIN study, 2010 ]. A similar study by Sorensen and colleagues showed a decrease relapse rate when methylprednisolone was used in combination with IFN-β1a subcutaneously compared with IFN-β1a alone [ Sorensen et al. 2009 ]. The advent of new immunomodulatory therapies and concerns for long-term adverse effects of steroids largely limit their use as a long-term preventative therapy.
What is in the pipeline?
At this time, six new drugs have entered or completed phase II and III clinical trials, three of which are oral drugs. These include laquinimod, teriflunomide and dimethyl fumarate, and three mAbs— alemtuzumab, daclizumab and rituximab.
Laquinimod is an orally administered immunomodulator being studied in patients with RRMS and SPMS. The anti-inflammatory properties of laquinimod are thought to be secondary to downregulation of MHC class II gene transcription factors, stimulation of neurotrophic factors, activation of the anti-inflammatory IL-4 pathway in CD4+ T cells, promotion of apoptosis in CD8+ T cells and B cells, and suppression of the metabolic activity of CD14+ and natural killer cells [ Thöne et al. 2011 ]. Therefore, it is proposed that laquinimod acts by affecting the Th1 to Th2 cytokine shift. Two phase II trials in patients with RRMS and SPMS have been completed, with varying results [ Comi et al. 2008 ; Polman et al. 2005 ]. In the phase III Assessment of Oral Laquinimod in Preventing Progression in Multiple Sclerosis (ALLEGRO study), laquinimod significantly improved clinical and radiologic outcomes, resulting in a 23% reduction in relapse rate, and a 37% reduction in mean cumulative number of Gad+ lesions [ Comi et al. 2012b ]. The Benefit Risk Assessment of Avonex and Laquinimod (BRAVO) study is another phase III study comparing laquinimod at 0.6 mg/day with weekly intramuscular IFN-β1a at 30 μg in patients with RRMS. At the 5th Joint Triennial Congress of the European and Americas Committees for Treatment and Research in Multiple Sclerosis (ECTRIMS/ACTRIMS), the results of a randomized, placebo-controlled, double-blind, active-comparator phase III study, BRAVO, were presented. This study did not achieve its primary endpoint of reducing the ARR. Laquinimod appears to be well tolerated, with only transient and dose-dependent increases in liver enzymes [ Vollmer et al. 2011 ].
Teriflunomide
Teriflunomide is an oral agent that inhibits the synthesis of DNA pyrimidine bases in rapidly dividing cells such as T and B cells and macrophages, and may thereby reduce inflammation (and likely produce immune suppression). It reversibly inhibits dihydroorotate dehydrogenase, a key enzyme involved in de novo pyrimidine synthesis. Because activated lymphocytes largely depend on de novo pyrimidine synthesis, pyrimidine depletion might result in inhibition of immune-cell proliferation [ Korn et al. 2004 ]. There is some evidence from in vitro studies suggesting that teriflunomide induces Th2-mediated anti-inflammatory cytokine activation. A phase II study examined the efficacy of teriflunomide daily doses of 7 and 14 mg compared with placebo over 36 weeks in patients with RRMS and SPMS. Teriflunomide efficacy was measured by the number of new lesions (T2 and Gad+) as observed on MRI scans. Active treatment resulted in a 61% reduction in MRI activity compared with placebo. Teriflunomide was generally well tolerated and occurrence of adverse events was similar between the two treatment groups. Serious adverse events included elevated liver enzyme levels, hepatic dysfunction, neutropenia, rhabdomyolysis and trigeminal neuralgia. A 2-year, double-blind, placebo-controlled phase III study in RRMS (The Teriflunomide Multiple Sclerosis Oral [TEMSO] study) was recently published. The study showed that at 7 and 14 mg the ARR was approximately 30% compared with placebo. Teriflunomide significantly reduced disability progression compared with placebo [ O’Connor et al. 2011 ].
Dimethyl fumarate
A novel oral therapy under development is dimethylfumaric acid (BG-12), which is related to fumaric acid, an agent used for many years in psoriasis (principally in Europe). BG-12 is thought to inhibit immune cells and molecules involved in MS attacks on the brain and spinal cord. Fumarates appear to modulate a number of oxidative pathways and thereby may influence the mechanisms by which autoimmune mechanisms provoke downstream pathways of tissue damage. In vitro studies with the ester dimethyl fumarate (DMF) described an inhibitory effect on nuclear factor κB dependent transcription of tumor necrosis factor α induced genes in human endothelial cells [ Moharregh-Khiabani et al. 2009 ]. Although its exact MOA is not known, BG-12 is thought to inhibit immune cells by stimulating the expression of anti-inflammatory cytokines, such as IL-10, IL-4 and IL-5. Hence, it is thought that DMF can induce a shift from a proinflammatory Th1 to an anti-inflammatory Th2 T-cell response [ Wierinckx et al. 2005 ]. In addition, BG-12 may have a neuroprotective therapeutic effect by inducing phase II detoxification genes and upregulation of the phase II detoxification enzyme, nicotinamide adenine dinucleotide phosphate oxidase:quinone oxidoreductase-1 [ Wierinckx et al. 2005 ]. In an earlier phase II study, compared with placebo, the BG-12 dose of 240 mg three times a day led to a 69% reduction in active inflammation on MRI scans from weeks 12 to 24 [ Kappos et al. 2008 ]. A phase III pivotal trial showed that 240 mg of BG-12, administered twice a day, met the primary study endpoint, demonstrating a highly statistically significant reduction in relapses in patients with RRMS, as well as providing a statistically significant reduction in ARR in the number of new or newly enlarging T2 hyperintense lesions, in new Gad+ lesions, and in the rate of disability progression at 2 years as measured by the EDSS [ Biogen Idec, 2011 ]. Side effects of BG-12 included abdominal pain, flushing, headache and fatigue [ Schimrigk et al. 2006 ].
Results of the phase III Efficacy and Safety of Oral BG00012 in Relapsing-Remitting Multiple Sclerosis (DEFINE) trial were presented at the 5th Joint Triennial Congress of the European and Americas Committees for Treatment and Research in Multiple Sclerosis (ECTRIMS/ACTRIMS). It showed that BG-12 at 24 mg twice or three times a day reduced the relapse rate by 49% and 50%, respectively.
Alemtuzumab
Alemtuzumab is a mAb that binds to CD52, an epitope common to most cells within the immune system. Treatment with this agent essentially results in an antibody-mediated ablation of the circulating immune system. Alemtuzumab binds to B and T lymphocytes, resulting in antibody-dependent cell lysis, and subsequent elimination from the bone marrow and blood, with the effect lasting up to 16 months. This agent appears to rapidly and profoundly establish both clinical and radiographic remission of MS; however, alemtuzumab has been associated with the risk of developing new autoimmune disorders (autoimmunity), including thyroiditis, idiopathic thrombocytopenic purpura and Goodpasture’s syndrome. Cossburn and colleagues found that the cumulative risk of autoimmunity in MS following the use of alemtuzumab was 22.2%, most frequent between 12 and 18 months following the first dose and evident for up to 5 years [ Cossburn et al. 2011 ].
Other adverse events associated with alemtuzumab include infections, increased cancer risk, organ toxicity and infusion-associated hypersensitivity reactions with potentially resultant neutralizing antibodies. Studies of alemtuzumab in the treatment of patients with RRMS and SPMS have suggested efficacy in the suppression of ARR, but with variable results in preventing progression of disability, depending on stages of the disease [ Corboy et al. 2010 ].
The phase II trial CAMMS223 compared alemtuzumab (12 or 24 mg intravenously on 5 consecutive days during the first month and on 3 consecutive days at months 12 and 24) with IFN-β1a, 44 μg subcutaneously three times a week. Alemtuzumab demonstrated a decrease in sustained disability (75% at 12 mg dose and 67% at 24 mg dose) and a decrease in relapse rate (69% at 12 mg and 79% at 24 mg) compared with IFN-β1a subcutaneously [ Panitch et al. 2008 ].
Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis Study 1 (CARE MS I), a 2 year phase III trial comparing alemtuzumab with subcutaneous IFN-β1a in treatment-naïve patients showed a reduction in relapse rate by 55% but did not show statistical significance regarding slowing disease process. A second phase III trial (CARE MS II) is currently in progress.
Daclizumab is an engineered human antibody that blocks the IL-2 receptor on immune cells. IL-2 is a potent immune stimulator and thus by blocking it, this therapy putatively dampens immune responses (including autoimmune responses).
The clinical benefit derived from daclizumab has been linked to the expansion of immunoregulatory CD56 natural killer cells, and the resulting downregulation of adaptive T-cell responses [ Bielekova et al. 2006 ]. A recent open-label phase II trial using subcutaneous daclizumab at 2 mg/kg in patients with MS whose condition showed an inadequate response to IFN-β therapy demonstrated a 72% reduction in the number of new or enlarged contrast-enhancing lesions at week 24 compared with patients receiving IFN-β alone [ Wynn et al. 2011 ]. A phase III trial of daclizumab versus IFN-β1a is ongoing (DECIDE study). Daclizumab is currently FDA approved for use in organ transplant patients.
Ocrelizumab
Ocrelizumab, the humanized version of rituximab, is an antibody that targets CD20, a cell surface epitope on developing B cells. Upon binding to its target, these agents provoke rapid destruction of circulating B cells via two principal mechanisms, antibody-dependent and complement-dependent cellular cytotoxicity.
The effect of CD20 targeting has ramifications for both B- and T-cell immune function, and as such, these treatments can be associated with risk of infection (including PML), and organ toxicity. Ocrelizumab, a humanized mAb against human CD20, is currently under investigation in a phase III program called ORCHESTRA (OPERA I and II in RRMS and ORATORIO in PPMS) evaluating its efficacy in patients with MS. A phase II trial comparing ocrelizumb 600 mg and 2000 mg with placebo showed a significant reduction in brain lesions (89% and 96%, respectively) and ARR (80% and 73%, respectively) [ Kappos et al. 2011 ].
The pharmacological armamentarium for MS has significantly expanded in the past 20 years and many new drugs are on the horizon. Each of these drugs has their own niche for utilization. There are significant data about long-term safety and efficacy of all the IFN-β and GA agents which should be considered first line in newly diagnosed patients. For patients whose condition fails to respond to first-line agents (i.e. recurrent clinical exacerbations, significant disease burden with new and enhancing lesions) or with an aggressive disease course at onset alternatives such as natalizumab or fingolimod are appropriate. MOAs, adverse events and compliance issues should be considered when choosing a therapy. Off-label agents also have a place for patients who are unable to tolerate FDA-approved therapies. These agents also offered options for intensification of therapy, although large clinical trials are needed to determine ideal dosing. The results of efficacy and safety profiles of emergent oral and intravenous agents are essential to determine their place in the treatment of patients with MS. With the advent of new therapies, the need for biomarkers that can predict a patient’s response to therapy is imperative. More studies are needed to develop therapies for halting neurodegeneration, promoting remyelination and promoting neuronal repair.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Wanda Castro-Borrero has received speaker honoraria from the National Multiple Sclerosis Society, Multiple Sclerosis Association of America, Teva Neuroscience and Biogen Idec. Donna Graves has received speaker honoraria from Teva Neuroscience, Bayer Pharmaceuticals and Novartis. Teresa C. Frohman has received speaker honoraria from Teva Neuroscience and Biogen Idec. Angela Bates has received speaker honoraria from the National Multiple Sclerosis Society, Multiple Sclerosis Association of America, Teva Neuroscience and Biogen Idec. Paula Hardeman has nothing to disclose. Diana Logan has received speaker honoraria from Teva Neuroscience, and consulting fees from Biogen Idec, Teva Neuroscience, Bayer Pharmaceuticals and Acorda Therapeutics. Megan Orchard has nothing to disclose. Benjamin Greenberg has received honoraria from EMD Serono, American Academy of Neurology, Multiple Sclerosis Association of America, and National Multiple Sclerosis Society, consulting fees from Acorda, DioGenix, Greater Good Foundation, and grants from Amplimmune, Accelerated Cure Project and Guthy Jackson Charitable Foundation. Elliot Frohman has received speaker fees from Biogen Idec, Teva Neuroscience, Acorda Pharmaceuticals, and consulting fees from Biogen Idec, Teva Neurosciences, Abbott, Acorda Therapeutics, and Novartis.
Contributor Information
Wanda Castro-Borrero, University of Connecticut Health Center, Neurology Associates, 263 Farmington Ave., Farmington, CT 06030-5357, USA.
Donna Graves, University of Texas Southwestern Medical Center, Multiple Sclerosis Program, Dallas, TX, USA.
Teresa C. Frohman, University of Texas Southwestern Medical Center, Multiple Sclerosis Program, Dallas, TX, USA
Angela Bates Flores, University of Texas Southwestern Medical Center, Multiple Sclerosis Program, Dallas, TX, USA.
Paula Hardeman, University of Texas Southwestern Medical Center, Multiple Sclerosis Program, Dallas, TX, USA.
Diana Logan, University of Texas Southwestern Medical Center, Multiple Sclerosis Program, Dallas, TX, USA.
Megan Orchard, University of Texas Southwestern Medical Center, Multiple Sclerosis Program, Dallas, TX, USA.
Benjamin Greenberg, University of Texas Southwestern Medical Center, Multiple Sclerosis Program, Dallas, TX, USA.
Elliot M. Frohman, University of Texas Southwestern Medical Center, Multiple Sclerosis Program, Dallas, TX, USA
- Achiron A., Gabbay U., Gilad R., Hassin-Baer R., Barak Y., Gornish M., et al. (1998) Intravenous immunoglobulin treatment in multiple sclerosis. Effect on relapses. Neurology 50: 398–402 [ DOI ] [ PubMed ] [ Google Scholar ]
- Achiron A., Kishner I., Sarova-Pinhas I., Raz H., Faibel M., Stern Y., et al. (2004) Intravenous immunoglobulin treatment following the first demyelinating event suggestive of multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Arch Neurol 61: 1515–1520 [ DOI ] [ PubMed ] [ Google Scholar ]
- Achiron A., Rotstein Z., Noy S., Mashiach S., Dulitzky M., Achiron R. (1996) Intravenous immunoglobulin treatment in the prevention of childbirth-associated acute exacerbations in multiple sclerosis: a pilot study. J Neurol 243: 25–28 [ DOI ] [ PubMed ] [ Google Scholar ]
- Aharoni R., Teitelbaum D., Sela M., Arnon R. (1997) Copolymer 1 induces T cells of the T helper type 2 that crossreact with myelin basic protein and suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 94: 10821–10826 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bar-Or A., Calabresi P.A., Arnold D., Markowitz C., Shafer S., Kasper L. H., et al. (2008) Rituximab in relapsing–remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol 63: 395–400 [ DOI ] [ PubMed ] [ Google Scholar ]
- Beck R.W., Cleary P.A., Trobe J. D., Kaufman D. I., Kupersmith M. J., Paty D. W., et al. (1993) The effect of corticosteroids for acute optic neuritis on the subsequent development of multiple sclerosis. The Optic Neuritis Study Group. N Engl J Med 329: 1764–1769 [ DOI ] [ PubMed ] [ Google Scholar ]
- Berger J.R. (2010) Progressive multifocal leukoencephalopathy and newer biological agents. Drug Saf 33: 969–983 [ DOI ] [ PubMed ] [ Google Scholar ]
- Bielekova B., Catalfamo M., Reichert-Scrivner S., Packer A., Cerna M., Waldmann T.A., et al. (2006) Regulatory CD56 (bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A 103: 5941–5946 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Biogen Idec (2011) Biogen Idec announces positive top-line results from the first phase III trial investigating oral BG-12 (dimethyl fumarate) in multiple sclerosis (press release). Weston, MA: Biogen Idec, 11 April 2011. Presented at 63rd annual meeting of the American Academy of Neurology, 9–16 April 2011, Honolulu, Hawaii [ Google Scholar ]
- Boster A., Ankeny D.P., Racke M.K. (2010) The potential role of B cell-targeted therapies in multiple sclerosis. Drugs 70: 2343–2356 [ DOI ] [ PubMed ] [ Google Scholar ]
- Casetta I., Iuliano G., Filippini G.(2009) Azathioprine for multiple sclerosis. J Neurol Neurosurg Psychiatry 80: 131–132; discussion 132 [ DOI ] [ PubMed ] [ Google Scholar ]
- Coelho R.P., Payne S.G., Bittman R., Spiegel S., Sato-Bigbee C. (2007) The immunomodulator FTY720 has a direct cytoprotective effect in oligodendrocyte progenitors. J Pharmacol Exp Ther 323: 626–635 [ DOI ] [ PubMed ] [ Google Scholar ]
- Cohen J.A., Barkhof F., Comi G., Hartung H.P., Khatri B.O., Montalban X., et al. for TRANSFORMS Study Group (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362: 402–415 [ DOI ] [ PubMed ] [ Google Scholar ]
- Comi G., De Stefano N., Freedman M.S., et al. (2012a) Comparison of two dosing frequencies of subcutaneous interferon beta-1a in patients with a first clinical demyelinating event suggestive of multiple sclerosis (REFLEX): a phase 3 randomised controlled trial. Lancet Neurol 11:33–41 [ DOI ] [ PubMed ] [ Google Scholar ]
- Comi G., Filippi M., Barkhof F., Durelli L., Edan G., Fernandez O., et al. (2001) Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet 357: 1576–1582 [ DOI ] [ PubMed ] [ Google Scholar ]
- Comi G., Jeffery D., Kappos L., Montalban X., Boyko A., Rocca M.A., et al. (2012b) Placebo controlled trial of oral laquinimod for multiple sclerosis. N Engl J Med 366: 1000–1009 [ DOI ] [ PubMed ] [ Google Scholar ]
- Comi G., Martinelli V., Rodegher M., Moiola M., Bajenaru O., Carra A., et al. for PreCISe Study Group (2009) Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet 374:1503–1511 [ DOI ] [ PubMed ] [ Google Scholar ]
- Comi G., Pulizzi A., Rovaris M., Abramsky O., Arbiizu T., Boiko A., et al. (2008) Effect of laquinimod on MRI-monitored disease activity in patients with relapsing–remitting multiple sclerosis: a multicentre, randomized, double-blind, placebo-controlled phase IIb study. The Lancet 371: 2085–2092 [ DOI ] [ PubMed ] [ Google Scholar ]
- Corboy J.R., Miravalle A. (2010) Emerging therapies for treatment of multiple sclerosis. J Inflamm Res 3: 53–39 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cossburn M., Pace A.A., Jones J., Ali R., Ingram G., Baker K., et al. (2011) Autoimmune disease after alemtuzumab treatment for multiple sclerosis in a multicenter cohort. Neurology 77: 573–579 [ DOI ] [ PubMed ] [ Google Scholar ]
- DiPiro J.T., Talbert R.L., Yee G.C., Matzke G.R., Wells B.G., Posey L.M. (2005) Pharmacotherapy, A Pathophysiologic Approach, 6th edition New York: McGraw-Hill [ Google Scholar ]
- Elovaara I., Apostolski S., van Doorn P., Gilhus N.E., Hietaharju A., Honkaniemi J., et al. (2008) EFNS guidelines for the use of intravenous immunoglobulin in treatment of neurological diseases: EFNS task force on the use of intravenous immunoglobulin in treatment of neurological diseases. Eur J Neurol 15: 893–908 [ DOI ] [ PubMed ] [ Google Scholar ]
- Fazekas F., Deisenhammer F., Strasser-Fuchs S., Nahler G., Mamoli B. (1997) Randomised placebo-controlled trial of monthly intravenous immunoglobulin therapy in relapsing–remitting multiple sclerosis. Austrian Immunoglobulin in Multiple Sclerosis Study Group. Lancet 349: 589–593 [ DOI ] [ PubMed ] [ Google Scholar ]
- Fazekas F., Lublin F.D., Freedman M.S., Hartung H.P., Rieckmann P., Sorensen P.S., et al. (2008) Intravenous immunoglobulin in relapsing–remitting multiple sclerosis: a dose-finding trial. Neurology 71: 265–271 [ DOI ] [ PubMed ] [ Google Scholar ]
- Ford C., Goodman A.D., Johnson K., Kachuck N., Lindsey J.W., Lisak R., et al. (2010) Continuous long-term immunomodulatory therapy in relapsing multiple sclerosis: results from the 15-year analysis of the US prospective open-label study of glatiramer acetate. Mult Scler 16: 342–350 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Foster C.A., Howard L.M., Schweitzer A., Persohn E., Hiestand P.C., Balatoni B., et al. (2007) Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J Pharmacol Exp Ther 323: 469–475 [ DOI ] [ PubMed ] [ Google Scholar ]
- Frohman E.M., Brannon K., Racke M.K., Hawker K. (2004) Mycophenolate mofetil in multiple sclerosis. Clin Neuropharmacol 27: 80–83 [ DOI ] [ PubMed ] [ Google Scholar ]
- Frohman E.M., Cutter G., Remington G., Gao H., Rossman H., Weinstock-Guttman B., et al. (2010) A randomized, blinded, parallel-group, pilot trial of mycophenolate mofetil (CellCept) compared with interferon beta-1a (Avonex) in patients with relapsing–remitting multiple sclerosis. Ther Adv Neurol Disord 3: 15–28 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Goodin D.S., Arnason B.G., Coyle P.K., Frohman E.M., Paty D.W. (2003) The use of mitoxantrone (Novantrone) for the treatment of multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 61: 1332–1338 [ DOI ] [ PubMed ] [ Google Scholar ]
- Gorelik L., Lerner M., Bixler S., Crossman M., Schlain B., Simon K., et al. (2010) Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol 68: 295–303 [ DOI ] [ PubMed ] [ Google Scholar ]
- Goverman J.M. (2011) Immune tolerance in multiple sclerosis. Immunol Rev 214: 228–240 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gray O., McDonnell G.V., Forbes R.B. (2004) Methotrexate for multiple sclerosis. Cochrane Database Syst Rev (2): CD003208. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Haas J., Hommes O.R. (2007) A dose comparison study of IVIG in postpartum relapsing–remitting multiple sclerosis. Mult Scler 13: 900–908 [ DOI ] [ PubMed ] [ Google Scholar ]
- Hartung H.P., Gonsette R., König N., Kwiecinski H., Guseo A., Morrissey S.P., et al. ; Mitoxantrone in Multiple Sclerosis Study Group (MIMS) (2002) Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double blind, randomized, multicentre trial. Lancet 360: 2018–2025 [ DOI ] [ PubMed ] [ Google Scholar ]
- Hauser S.L., Waubant E., Arnold D.L., Vollmer T., Antel J., Fox R.J., et al. (2008) B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N Engl J Med 358: 676–688 [ DOI ] [ PubMed ] [ Google Scholar ]
- Hawker K., O’Connor P., Freedman M.S., Calabresi P.A., Antel J., Simon J., et al. (2009) Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol 66: 460–471 [ DOI ] [ PubMed ] [ Google Scholar ]
- IFNB Multiple Sclerosis Study Group (1993) Interferon beta-1b is effective in relapsing–remitting multiple sclerosis. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 43: 655–661 [ DOI ] [ PubMed ] [ Google Scholar ]
- Jacobs L.D., Beck R.W., Simon J.H., Kinkel R.P., Brownscheidle C.M., Murray T.J., et al. (2000) Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. N Engl J Med 343: 898–904 [ DOI ] [ PubMed ] [ Google Scholar ]
- Jacobs L.D., Cookfair D.L., Rudick R.A., Herndon R.M., Richert J.R., Salazar A.M., et al. (1996) Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG) Ann Neurol 39: 285–294 [ DOI ] [ PubMed ] [ Google Scholar ]
- Johnson K.P. (2010) The Remarkable Story of Copaxone. New York: Diamedica, pp. 70–73, 196–199 [ Google Scholar ]
- Kappos L., Freedman M.S., Polman C.H., Edan G., Hartung H.P., Miller D.H., et al. (2009) Long term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol 8: 987–997 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kappos L., Gold R., Miller D.H., Macmanus D.G., Havrdova E., Limmroth V., et al. (2008) Efficacy and safety of oral fumarate in patients with relapsing–remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 372: 1463–1472 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kappos L., Li D., Calabresi P.A., O’Connor P., Bar-Or A., Barkhof F., et al. (2011) Ocrelizumab in relapsing–remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. The Lancet 378: 1779–1787 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kappos L., Lindberg R.L.P. (2007) Interferons in relapsing–remitting multiple sclerosis. In: Cohen J.A., Rudick R.A. (eds), Multiple Sclerosis Therapeutics, 3rd edition London: Informa Healthcare, pp. 373–392 [ Google Scholar ]
- Kappos L., Polman C.H., Freedman M.S., Edan G., Hartung H.P., Miller D.H., et al. (2006) Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 67: 1242–1249 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kappos L., Radue E.W., O’Connor P., Polman C., Hohlfeld R., Calabresi P., et al. for the FREEDOMS Study Group (2010) A placebo controlled-trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362: 387–401 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kieseier B.C. (2011) The mechanism of action of interferon-β in relapsing multiple sclerosis. CNS Drugs 25: 491–502 [ DOI ] [ PubMed ] [ Google Scholar ]
- Korn T., Magnus T., Toyka K., Jung S. (2004) Modulation of effector cell functions in experimental autoimmune encephalomyelitis by leflunomide – mechanisms independent of pyrimidine depletion. J Leukoc Biol 76: 950–960 [ DOI ] [ PubMed ] [ Google Scholar ]
- Lalive P.H., Neuhaus O., Benkhoucha M., Burger D., Hohlfeld R., Zamvil S.S., et al. (2011) Glatiramer acetate in the treatment of multiple sclerosis emerging concepts regarding its mechanism of action. CNS Drugs 25: 401–414 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- La Mantia L., Mascoli N., Milanese C. (2007) Azathioprine. Safety profile in multiple sclerosis patients. Neurol Sci 28(6): 299–303 [ DOI ] [ PubMed ] [ Google Scholar ]
- Lewanska M., Siger-Zajdel M., Selmaj K. (2002) No difference in efficacy of two different doses of intravenous immunoglobulins in MS: clinical and MRI assessment. Eur J Neurol 9: 565–572 [ DOI ] [ PubMed ] [ Google Scholar ]
- Marriott J.J., Miyasaki J.M., Gronseth G., O’Connor P.W. (2010) Evidence report: the efficacy and safety of mitoxantrone (Novantrone) in the treatment of multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 74: 1463–1470 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Martinelli V., Radaelli M., Straffi L., Rodegher M., Comi G. (2009) Mitoxantrone: benefits and risks in multiple sclerosis patients. Neurol Sci 30(Suppl. 2): S167–S170 [ DOI ] [ PubMed ] [ Google Scholar ]
- McDonagh M., Dana T., Chan B.K.S., Thakurta S., Gibler A. (2007) Drug class review on disease-modifying drugs for multiple sclerosis: final report (internet). Portland, OR: Oregon Health & Science University; Available at: http://www.ncbi.nlm.nih.gov/books/NBK10578/ (last accessed July 2007). [ PubMed ] [ Google Scholar ]
- Mikol D.D., Barkhof F., Chang P., Coyle P.K., Jeffery D.R., Schwid S.R., et al. (2008) Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol 7: 903–914 [ DOI ] [ PubMed ] [ Google Scholar ]
- Miron V.E., Jung C.G., Kim H.J., Kennedy T.E., Soliven B., Antel J.P. (2008) FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann Neurol 63: 61–71 [ DOI ] [ PubMed ] [ Google Scholar ]
- Moharregh-Khiabani D., Linker R.A., Gold R., Stangel M. (2009) Fumaric acid and its esters: an emerging treatment for multiple sclerosis. Curr Neuropharmacol 7: 60–64 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- O’Connor P., Filippi M., Arnason B., Comi G., Cook S., Goodin D., et al. (2009) Interferon beta-1b 500 mcg, interferon beta-1b 250 mcg and glatiramer acetate: primary outcomes of the BEYOND (Betaferon/Betaseron Efficacy Yielding Outcomes of New Dose) study. Lancet Neurol 8: 889–897 [ DOI ] [ PubMed ] [ Google Scholar ]
- O’Connor P., Wolinsky J., Confavreux C., Comi G., Kappos L., Olsson T.P., et al. for the TEMSO Trial Group (2011) Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 365: 1293–1303 [ DOI ] [ PubMed ] [ Google Scholar ]
- Panitch H., Coles A.J., Compston P.A., Selmaj K.A., Lake S.L., Moran S., et al. for CAMMS223 (2008) Alemtuzumab vs interferon B-1a in early multiple sclerosis. N Engl J Med 359: 1786–1801 [ DOI ] [ PubMed ] [ Google Scholar ]
- Polman C.H., Barkhof F., Sandberg-Wollheim M., Linde A., Nordle O., Nederman T., et al. (2005) Treatment with laquinimod reduces development of active MRI lesions in relapsing multiple sclerosis. Neurology 64: 987–991 [ DOI ] [ PubMed ] [ Google Scholar ]
- Polman C.H., Bertolotto A., Deisenhammer F., Giovannoni G., Hartung H.P., Hemmer B., et al. (2010) Recommendations for clinical use of data on neutralizing antibodies to interferon-beta therapy in multiple sclerosis. Lancet Neurol 9: 740–750 [ DOI ] [ PubMed ] [ Google Scholar ]
- Polman C.H., O’Connor PW., Havrdova E., Hutchinson M., Kappos L., Miller D.H., et al. (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354: 899–910 [ DOI ] [ PubMed ] [ Google Scholar ]
- Prescribing information (2010) RITUXAN ® (rituximab). San Francisco: Genentech [ Google Scholar ]
- Prevention of Relapses and Disability by Interferon beta 1-a Subcutaneously in Multiple Sclerosis (PRISMS) Study Group and Ebers, G.C. (1998) Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 352: 1498–1504 [ PubMed ] [ Google Scholar ]
- Product information: Cellcept (2009) Nutley, NJ: Roche Laboratories [ Google Scholar ]
- Product information: methotrexate oral tablet (2000) Columbus, OH: Roxane Laboratories [ Google Scholar ]
- Product information: methotrexate injections (2005) USP, methotrexate sodium. Paramus, NJ: Mayne Pharma [ Google Scholar ]
- Ransohoff R.M. (2007) Natalizumab for multiple sclerosis. N Engl J Med 356: 2622–2629 [ DOI ] [ PubMed ] [ Google Scholar ]
- Ravnborg M., Sorensen P.S., Andersson M., Celius E.G., Jongen P.J., Elovaara I., et al. (2010) Methylprednisolone in combination with interferon beta-1a for relapsing remitting multiple sclerosis (MECOMBIN study): a multicentre, double-blind, randomised, placebo-controlled, parallel-group trial. Lancet Neurology 9: 672–680 [ DOI ] [ PubMed ] [ Google Scholar ]
- Rudick R.A., Stuart W.H., Calabresi P.A., Confavreux C., Galetta S.L., Radue E.W., et al. (2006) Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 354: 911–923 [ DOI ] [ PubMed ] [ Google Scholar ]
- Schimrigk S., Brune N., Hellwig K., Lukas C., Bellenberg B., Rieks M., et al. (2006) Oral fumaric acid esters for the treatment of active multiple sclerosis: an open-label, baseline-controlled pilot study. Eur J Neurol 13(6): 604-610 [ DOI ] [ PubMed ] [ Google Scholar ]
- Sorensen P.S., Mellgren S.I., Svenningsson A., Elovaara I., Frederiksen J.L., Beiske A.G., et al. (2009) NORdic trial of oral Methylprednisolone as add-on therapy to Interferon beta-1a for treatment of relapsing–remitting Multiple Sclerosis (NORMIMS study): a randomised, placebo-controlled trial. Lancet Neurol 8: 519–529 [ DOI ] [ PubMed ] [ Google Scholar ]
- Sorensen P.S., Bertolotto A., Edan G., Giovannoni G., Gold R., Havrdova E., et al. (2012) Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumb. Mult Scler 18: 143–152 [ DOI ] [ PubMed ] [ Google Scholar ]
- Thöne J., Gold R. (2011) Laquinimod: a promising oral medication for the treatment of relapsing–remitting multiple sclerosis. Expert Opin Drug Metab Toxicol 7: 365–370 [ DOI ] [ PubMed ] [ Google Scholar ]
- Vollmer T.L., Sorensen P.S., Arnold D.L. MS-LAQ-302 Benefit Risk Assessment of Avonex and Laquinimod (BRAVO) Study Group (2011) A placebo-controlled and active comparator phase iii trial of oral laquinimod in relapsing–remitting multiple sclerosis patients, Bittman, R., Spiegel, S. and Sato-Bigbee, C. ( NCT00605215 ). Abstract 148 Presented at: 5th Joint Triennial Congress of the European and Americas Committees for Treatment and Research in Multiple Sclerosis (ECTRIMS/ACTRIMS), October 2011 [ Google Scholar ]
- Wierinckx A., Breve J., Mercier D., Schultzberg M., Drukarch B., Van Dam A.M. (2005) Detoxication enzyme inducers modify cytokine production in rat mixed glial cells. J Neuroimmunol 166: 132–143 [ DOI ] [ PubMed ] [ Google Scholar ]
- Wynn D., Kaufman M., Montalban X., Vollmer T., Simon J., Elkins J., et al. (2011) Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon beta. Lancet Neurol 9: 381–390 [ DOI ] [ PubMed ] [ Google Scholar ]
- Zivadinov R., Rudick R.A., De Masi R., Nasuelli D., Ukmar M., Pozzi-Mucelli R.S., et al. (2001) Effects of IV methylprednisolone on brain atrophy in relapsing–remitting MS. Neurology 57: 1239–1247 [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (428.7 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Research at Microsoft 2024: Meeting the challenge of a changing world
We live in a rapidly changing world..
Demographic shifts, climate change, political dynamics, and many other forces are creating urgent challenges in critical areas like global health and scientific discovery, environmental sustainability, food security, and societal resilience.
In this new AI era, technology is changing even faster than before, and the transition from research to reality, from concept to solution, now takes days or weeks rather than months or years.
“Today we are seeing so much AI research happening at the speed of conversation, to the point where even our top researchers feel that their heads are spinning, but working together, providing openness, providing greater access, we can see that we’ve made tremendous progress.” – Peter Lee, President, Microsoft Research
In 2024, Microsoft Research continued its foundational research (opens in new tab) to expand the capabilities of large language models, but we also explored more deeply how smaller models (opens in new tab) can be trained for specific tasks. We discovered that by using smaller datasets and fewer compute resources, some small language models can demonstrate enhanced reasoning and other complex capabilities that were once considered the exclusive province of large-scale models.
October 2024 | LinkedIn
Microsoft Research and its external collaborators used AI to enable earlier detection and treatment of esophageal cancer , which could lead to dramatically improved survival rates, and to accelerate the discovery of new drugs needed to treat infectious diseases that kill millions of people every year. And we continued to use AI to develop new tools for scientific discovery so that we and others in the scientific community can confront some of humanity’s most important challenges.
One team of Microsoft researchers created the world’s first large-scale model of the atmosphere , which could transform weather forecasting and our ability to predict and mitigate the effects of extreme weather events. Another team worked with global experts to create a generative AI tool that empowers non-governmental organizations (NGOs) to fight human trafficking .
We also opened a new research lab in Tokyo this year. It joins our other labs in Europe, Asia, Africa, and North America. And we launched a series of quarterly Research Forums to help update the global research community about some of the pivotal work we’re doing at Microsoft Research. Register for future episodes , view presentations from previous forums, and explore our briefing book content .
This post highlights some of the work that Microsoft Research has done in 2024, along with academic and industry colleagues, to help drive real-world benefits for people worldwide.
AI for Business Transformation: Multimodal Models
Top social posts of 2024.

Accelerating Foundation Models Research
Microsoft Research program encourages AI development by academics, not just industry.
Ece Kamar on the future of AI agents

Introducing BitNet b1.58
1.58-bit LLMs that rival full-precision Transformer LLMs in performance while significantly boosting efficiency—in terms of latency, throughput, memory and energy consumption.
A small language model that performs as well as (and often better than) large models on certain types of complex reasoning tasks.
A breakthrough in training fully sparsely-activated LLMs supports both full-precision and 1-bit LLMs.
You Only Cache Once (YOCO)
A novel decoder-decoder architecture for LLMs, enhancing memory efficiency by caching key-value pairs only once.
Differential Transformer
A new foundation architecture for LLMs that enhances focus on relevant information while canceling attention noise.
AI Controller Interface
Helping researchers and developers efficiently implement existing strategies for controlling LLMs and invent new ones.
GraphRAG 1.0
Advancing AI use in complex domains like science.
MatterSimV1
A deep learning atomistic model across elements, temperatures, and pressures.
Top stories of 2024
Ghddi and microsoft research use ai to achieve progress in new drug discovery for global infectious diseases.

The joint team designed several chemical compounds that are effective in inhibiting these pathogens’ essential target proteins, accelerating the structure-based drug discovery process.

Microsoft announced the 10 inaugural grant recipients through the Accelerate Foundation Models Research Minority Serving Institutions grant program.

Leading researchers at Microsoft explored the latest breakthroughs in AI models, new applications of AI to important scientific challenges, novel approaches to model evaluation and understanding, and other key research topics.

The work shows that the removal of certain parameters not only maintains model performance like some existing parameter-reduction methods but can actually improve it—no additional training necessary.
GraphRAG: Unlocking LLM discovery on narrative private data

GraphRAG is a significant advance in enhancing the capability of LLMs and enables us to answer important classes of questions that we cannot attempt with baseline RAG alone.

ViSNet emerged as a versatile tool capable of giving insight into the intricate relationships between molecular structure and biological activity.
Can how we think about our thinking help us better incorporate generative AI into our lives and work? Microsoft researchers explored metacognition’s potential to improve the tech’s usability.
This year, AI is expected to become more accessible, nuanced, and integrated in technologies that improve everyday tasks and help solve some of the world’s most challenging problems.
Scaling early detection of esophageal cancer with AI

Our collaboration with Cyted demonstrates the transformative potential of integrating advanced AI models into clinical workflows. Earlier detection of cancer and earlier start of treatment mean that more than 9 in 10 patients survive 5 years after diagnosis.

By training Orca-Math on a small dataset of 200,000 math problems, we have achieved performance levels that rival or surpass those of much larger models.

We hope to enable the developer community to benefit from the cache-store system’s performance gains and capabilities, to build on our work, and to expand the Garnet ecosystem by adding new API calls and features.

Leading researchers at Microsoft discussed how AI is transforming health care and the natural sciences, the intersection of AI and society, and the continuing evolution of foundational AI technologies.
Senior Researcher Chang Liu discusses M-OFDFT, which leverages deep learning to help identify molecular properties in a way that minimizes the tradeoff between accuracy and efficiency, work with the potential to benefit drug and materials discovery.

Principal Researcher Ida Momennejad brings her expertise in cognitive neuroscience and computer science to this in-depth conversation about general intelligence and what the evolution of the brain across species can teach us about building AI.
SIGMA: An open-source mixed-reality system for research on physical task assistance

Imagine if every time you needed to complete a complex physical task you had a world-class expert standing over your shoulder and guiding you through the process. What would it take to build an interactive AI system that could assist you with any task in the physical world?

SAMMO streamlined the optimization of prompts, particularly those that combine different types of structural information, to enable AI practitioners and researchers to efficiently refine prompts with little manual effort.

Researchers proposed a method for leveraging the motion of small satellites to facilitate efficient communication between a large IoT-satellite constellation and devices on Earth within a limited spectrum.

NSDI provides a platform for researchers and experts to share insights, present research findings, and collaborate on the latest advances in the design, implementation, and evaluation of networked and distributed systems.

ASPLOS is the main forum where researchers bridge the gap between architecture, programming languages, and operating systems to advance the state of the art.

The new series “Ideas” debuts with guest Kalika Bali. The speech and language tech researcher talks sci-fi and its impact on her career, the design thinking philosophy behind her research, and the “outrageous idea” she had to work with low-resource languages.
MatterSim: A deep-learning model for materials under real-world conditions

The model efficiently handles simulations for a variety of materials, including metals, oxides, sulfides, halides, and their various states such as crystals, amorphous solids, and liquids.

Breakthroughs in robotics technology are poised to drive productivity, efficiency, and innovation across numerous industries. RASCAL improves the efficiency of vertical storage systems by operating across evenly spaced, parallel shelves and horizontal rails.

The confluence of digital transformation in biomedicine and the current generative AI revolution creates an unprecedented opportunity for drastically accelerating progress in precision health. Prov-GigaPath attains state-of-the-art performance on standard cancer classification and pathomics tasks, as well as vision-language tasks.

LoftQ adapts pre-trained language models to perform well in specialized applications. During fine-tuning, the model undergoes additional training on a smaller, task-specific dataset for improved performance.

FastGen optimizes the way LLMs store and access data, potentially cutting memory use by half while preserving their efficiency.
Aurora: The first large-scale foundation model of the atmosphere

Aurora presents a new approach to weather forecasting that could transform our ability to predict and mitigate the impacts of extreme events. The model can forecast a broad range of atmospheric variables, from temperature and wind speed to air-pollution levels and concentrations of greenhouse gases.

AutoGen Studio is the next step forward in enabling developers to advance the multi-agent paradigm.

Leading researchers at Microsoft dove into the importance of globally inclusive and equitable AI, shared updates on AutoGen and MatterGen, explored novel use cases for AI, and more.

The conference brings together experts from a wide range of disciplines who are committed to the responsible development of computational systems.

This conference covered a broad spectrum of topics, including 3D reconstruction and modeling, action and motion analysis, video and image processing, synthetic data generation, neural networks, and more.
Data-driven model improves accuracy in predicting EV battery degradation

Microsoft Research collaborated with Nissan Motor Corporation to develop a new machine learning method that predicts battery degradation with an average error rate of just 0.94%, significantly bolstering Nissan’s battery recycling efforts.

Intelligence Toolkit is a human rights technology developed with global experts in the anti-trafficking community, yet applicable to a broad class of problems impacting societal resilience as a whole.

Trace is a new AutoDiff-like tool for training AI systems without using gradients. This generalization is made possible by OPTO, which can describe end-to-end optimization of AI systems with general feedback.

Researchers introduced an automated multi-agent framework for creating diverse, high-quality synthetic data at scale for language model post-training.

In an era increasingly steered by data, machine learning is a pivotal force, transforming vast amounts of information into actionable intelligence with unprecedented speed and accuracy.
Large-scale pathology foundation models show promise on a variety of cancer-related tasks

Imagine if pathologists had tools that could help predict therapeutic responses just by analyzing images of cancer tissue. By leveraging AI and machine learning, researchers are now able to analyze digitized tissue samples with unprecedented accuracy and scale, potentially transforming how we understand and treat cancer.

With the skilled input of experienced game designers, tools like GENEVA could increasingly contribute to creating engaging gameplay experiences.

A multidisciplinary research team is exploring one solution: a credential that allows people to show they’re not bots without sharing identifying information.
AI has not yet delivered its full economic potential. Researchers at Microsoft are working to address the challenges that hold back progress.
Find My Things: New teachable AI tool helps blind and low-vision people locate lost personal items

Find My Things makes it easy for people with vision disabilities to use their phones to recognize and locate the personal items they use every day.

MedFuzz is an adversarial machine learning method designed to reveal how much benchmark performance relies on unrealistic assumptions.

Eureka is an open-source framework for standardizing evaluations of large foundation models beyond single-score reporting and rankings.

College freshman Dexter Greene and Microsoft research manager Richard Black discuss how technology that stores data in glass is supporting students as they expand earlier efforts to communicate what it means to be human to extraterrestrials.

Leading researchers at Microsoft shared the latest multimodal AI models, advanced benchmarks for AI evaluation and model self-improvement, and an entirely new kind of computer for AI inference and hard optimization.
Data Formulator: Exploring how AI can help analysts create rich data visualizations

Data Formulator’s architecture separates data transformation from chart configuration, improving both the user experience and AI performance. Refining how users interact with AI-powered tools is essential for improving how they communicate their requirements, paving the way for more efficient and effective collaboration.

DRIFT Search introduces a new approach to local search queries by including community information in the search process.
The combination of Novo Nordisk’s industry-leading domain expertise and Microsoft’s industry-leading applied AI expertise is opening new and exciting possibilities to shape the future of life sciences.
Microsoft researchers are working to apply foundation models—large-scale models that take advantage of recent AI advances—to scientific disciplines.
Peter Lee discusses what’s next for AI in this GeekWire podcast. Peter’s comments begin at 29:20.
Since the early 1990s, the promise of AI has been a driving force at Microsoft Research, which has a track record of breakthroughs that continue to advance the state of the art.
Microsoft establishes a new lab, Microsoft Research Asia – Tokyo (opens in new tab)

The Tokyo lab will focus on critical areas that reflect Japan’s socioeconomic priorities, including embodied AI, well-being and neuroscience, societal AI, and industry innovation. These research efforts aim to leverage advanced technologies to foster societal progress and contribute to the region’s innovation ecosystem.

Microsoft Research has been working on the development of efficient methods aiming for ab initio accuracy simulations of biomolecules. This method, AI 2 BMD ( AI -based a b i nitio b io m olecular d ynamics system), published in the journal Nature, represents the culmination of a four-year research endeavor.

By unifying object recognition, detection, and segmentation into a single framework, BiomedParse allows users to specify what they’re looking for through a simple, natural-language prompt.

Researchers Chris Hawblitzel and Jay Lorch share how progress in programming languages and verification approaches are bringing bug-free software within reach. Their work on the Rust verification tool Verus won the Distinguished Artifact Award at SOSP ’24.

TamGen offers a new approach to drug discovery by applying the principles of generative AI to molecular design.

In an age where digital infrastructure underpins nearly every facet of modern life, SOSP serves as an important forum for showcasing the technologies that shape our interconnected world.

Research manager Karin Strauss and members of the DNA Data Storage Project reflect on the path to developing a synthetic DNA–based system for archival data storage, including the recent open-source release of its most powerful algorithm for DNA error correction.
MarS: A unified financial market simulation engine in the era of generative foundation models

These innovations have the potential to empower financial researchers to customize generative models for diverse scenarios, establishing a new paradigm for applying generative models to downstream tasks in financial markets.
Learn about Phi-4, the latest small language model in Phi family, that offers high-quality results at a small size (14B parameters).

More than 100 papers by Microsoft researchers and collaborators have been accepted at NeurIPS 2024, including five oral presentations and 19 spotlight sessions.

PromptWizard from Microsoft Research is now open source. It is designed to automate and simplify AI prompt optimization, combining iterative LLM feedback with efficient exploration and refinement techniques to create highly effective prompts in minutes.

As the “biggest election year in history” comes to an end, researchers Madeleine Daepp and Robert Osazuwa Ness and Democracy Forward GM Ginny Badanes discuss AI’s impact on democracy, including Daepp and Ness’s research into the tech’s use in Taiwan and India.
According to Ashley Llorens, corporate vice president and managing director at Microsoft Research, AI models will soon be able to handle far more complex tasks.
Thank you for reading, watching, and listening
In 2024, contributions across the research community at Microsoft continued to advance the company’s vision of what technology can and should be: a means for empowering every person and every organization on the planet to achieve more.
To stay informed of the latest innovations, subscribe to the Microsoft Research Newsletter (opens in new tab) and the Microsoft Research Podcast (opens in new tab) . You can also follow us on Bluesky (opens in new tab) , Facebook (opens in new tab) , Instagram (opens in new tab) , LinkedIn (opens in new tab) , X (opens in new tab) , and YouTube (opens in new tab) .
Writers, Editors, and Producers : Kristina Dodge, Kate Forster, Jessica Gartner, Alyssa Hughes, Gretchen Huizinga, Lindsay Kalter, Brenda Potts, Chris Stetkiewicz, Larry West
Media Producers : Jeremy Crawford, Chris Duryee, Ben Ericson, Jeremy Mashburn, Matthew McGinley, Wil Morrill, Sandro Nigris, Joe Plummer, Craig Tuschhoff
Managing Editor, Research Publishing : Amber Tingle
Project Manager : Amanda Melfi
Microsoft Research Global Design Lead : Neeltje Berger
Graphic Designers : David Celis Garcia, Tetiana Buhinskaya
Director, Microsoft Research Creative Studio : Matt Corwine
Other resources
Microsoft Research Podcast
Microsoft Research Blog
Microsoft Research Forum series
- Follow on X
- Like on Facebook
- Follow on LinkedIn
- Subscribe on Youtube
- Follow on Instagram
- Subscribe to our RSS feed
Share this page:
- Share on Facebook
- Share on LinkedIn
- Share on Reddit

IMAGES
COMMENTS
Stay informed on the latest breakthroughs and developments in MS research and discover the historical milestones we have made in research progress for MS.
Stay informed on the latest breakthroughs and developments in MS research and discover the historical milestones we have made in research progress for MS.
1 day ago · The team at Multiple Sclerosis News Today has brought our audience the latest news about treatments, scientific research, and clinical trials in multiple sclerosis (MS) throughout 2024. Here is a ...
Get up-to-date with everything MS research. Our research community keep us updated with all the latest news. You can also head over to our research blog for a more in depth look at developments. Search for news...
3 days ago · 3. Phase 3 DAYBREAK Trial Highlights Long-Term Efficacy of Ozanimod for Relapsing Multiple Sclerosis. New long-term data from the phase 3 open-label extension DAYBREAK trial (NCT02576717) presented at the 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum, February 29 to March 2, in West Palm Beach, Florida, showed sustained efficacy for measures of ...
Nov 22, 2024 · The world’s leading experts in multiple sclerosis (MS) gathered in Copenhagen, Denmark in September 2024 to share the results of their latest research into the causes, course, and treatment of the disease.
Aug 6, 2024 · Scientists at UCSF and Contineum Therapeutics have developed a drug that spurs the body to replace the protective insulation around nerve cells, myelin, that is lost in multiple sclerosis. If it works in people, it could be a way to reverse the damage caused by the disease.
Today, clinical studies like those being conducted by the UCSF Weill Institute for Neurosciences, which Hauser directs, are investigating new ways to aggressively treat MS sooner with existing medications and new, more powerful versions.
Dec 25, 2024 · Information on multiple sclerosis. Read current research articles and learn about multiple sclerosis diagnosis, symptoms as well as the latest MS treatment options.
Dec 16, 2024 · Multiple sclerosis is an autoimmune disease in which immune cells attack and destroy the protective myelin sheaths that surround nerve fibres, leading to neurological disturbances. The immune...
4 days ago · A total of 179 patients with MS treated with IRT were included in the study. All patients had RMS. Thirty-seven patients (20.7%) were treated with AHSCT, 19 patients (10.6%) were treated with ALE ...
Sep 3, 2024 · The Multiple Sclerosis Journal has published the updated Pathways to Cures Roadmap accounting for recent research and scientific advances towards a cure for MS. Updated Roadmap defines current research priorities based on new knowledge of the disease; is endorsed by 30 MS organizations worldwide.
A Promising Candidate to Promote Neuroprotection and Repair Processes in MS. Dr. Soheila Karimi’s (University of Manitoba) research, which we funded, found a potential treatment, called Neuregulin-1, that could help repair the protective covering of nerve fibres and prevent further damage. In this new study, the research team will use mice with MS-like disease to test how effective ...
Jun 28, 2023 · A large, collaborative study on multiple sclerosis (MS) severity found that a single gene variant is predictive of much faster neurodegeneration in MS patients.
Feb 21, 2024 · Treatment options for progressive types of multiple sclerosis (MS) have expanded dramatically over the past decade, and several promising experimental therapies are in late stages of clinical...
Stay up to date with the latest news, breakthroughs, and advancements on MS treatments, related legislation, Society updates, and more in the MS community.
Nov 1, 2024 · Researchers continue to search for new possibilities to cure MS—or at least better ways to manage the disease. Here are seven in the works.
May 22, 2024 · Research into new MS treatments is ongoing. While there’s no cure yet, the scientific study of MS has led to significant breakthroughs. Read on to learn more.
Read about the latest MS research breakthroughs. Go behind the headlines with insights from our scientists and research team. How are treatments developed? It takes time to go from a scientific idea to a new treatment. Find out about the steps involved and why they are important. What is good research? Not all research is equal.
May 30, 2022 · What is the most recent research on multiple sclerosis, and are there any new treatments on the horizon? We spoke to three experts to find out more.
2 days ago · The therapeutic management of relapsing multiple sclerosis (RMS) typically includes two strategies: escalation and induction [1,2]. The escalation strategy involves the use of first-line disease-modifying therapies (DMTs) and, if these fail, switching to a more aggressive treatment.
Get the latest multiple sclerosis news, patient views, and caregiver perspectives from one of the largest MS communities online.
Find out about opportunities to join the movement, the latest breakthroughs and how they can help you live your best life with MS. We are the driving force of MS research and treatment to stop disease progression, restore function, and end MS forever. Support our progress towards a cure.
Mar 29, 2021 · In this Special Feature, learn about recent and ongoing research that sheds light on the causes of MS and reveals potential new avenues of treatment.
Patients receiving IFN-β1b were also found to have a lower T2 lesion volume and decreased accumulation of new lesions [IFNB Multiple Sclerosis Study Group. 1993 ... At the 5th Joint Triennial Congress of the European and Americas Committees for Treatment and Research in Multiple Sclerosis (ECTRIMS/ACTRIMS), the results of a randomized, placebo ...
However, up to 10% of all patients with MS are younger than 18 years at first manifestation, and less than 1% are younger than 10 years. 1 A growing body of research suggests that signs or symptoms nonspecific for MS manifest years before disease onset, suggestive of a prodromal phase. 2,3 For example, a Canadian study showed that patients with ...
Microsoft Research and its external collaborators used AI to enable earlier detection and treatment of esophageal cancer, which could lead to dramatically improved survival rates, and to accelerate the discovery of new drugs needed to treat infectious diseases that kill millions of people every year. And we continued to use AI to develop new tools for scientific discovery so that we and others ...