Heavy metals in vegetables: a review of status, human health concerns, and management options
Affiliations.
- 1 Department of Life Sciences, Vivekananda Global University, Jaipur, Rajasthan, 303012, India.
- 2 Department of Zoology, Delhi University, Delhi, 110007, India.
- 3 School of Forensic Science, National Forensic Science University, Gandhinagar, 382007, India.
- 4 Central Laboratory, Rajasthan State Pollution Control Board, Jaipur, Rajasthan, 302004, India.
- 5 Department of Life Sciences, Vivekananda Global University, Jaipur, Rajasthan, 303012, India. [email protected].
- PMID: 35921005
- DOI: 10.1007/s11356-022-22210-w
For sustainable global growth, food security is a prime concern issue, both quantitatively and qualitatively. Adverse effects on crop quality from contaminants like heavy metals have affected food security and human health. Vegetables comprise the essential and nutritious part of the human diet as they contain a lot of health-promoting minerals and vitamins. However, the inadvertent excess accumulation of heavy metals (As, Cd, Hg, and Pb) in vegetables and their subsequent intake by humans may affect their physiology and metabolomics and has been associated with diseases like cancer, mental retardation, and immunosuppression. Many known sources of hazardous metals are volcano eruptions, soil erosion, use of chemical fertilizers in agriculture, the use of pesticides and herbicides, and irrigation with wastewater, industrial effluents, etc. that contaminate the vegetables through the soil, air and water. In this review, the problem of heavy metal contamination in vegetables is discussed along with the prospective management strategies like soil amendments, application of bioadsorbents, membrane filtration, bioremediation, and nanoremediation.
Keywords: Contamination; Health impact and management; Heavy metals; Vegetables.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.

Publication types
- Agriculture
- Environmental Monitoring
- Metals, Heavy* / analysis
- Prospective Studies
- Soil Pollutants* / analysis
- Metals, Heavy
- Soil Pollutants
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Accumulation of Heavy Metals in Vegetable Species Planted in Contaminated Soils and the Health Risk Assessment
Wen-tao yang, jiao-feng gu, wen-lei wang, jia-ling zou, pei-qin peng, bo-han liao.
- Author information
- Article notes
- Copyright and License information
Correspondence: [email protected] ; Tel.: +86-073-189-814-019
Received 2016 Jan 14; Accepted 2016 Feb 18; Issue date 2016 Mar.
This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/ ).
The objectives of the present study were to investigate heavy metal accumulation in 22 vegetable species and to assess the human health risks of vegetable consumption. Six vegetable types were cultivated on farmland contaminated with heavy metals (Pb, Cd, Cu, Zn, and As). The target hazard quotient (THQ) method was used to assess the human health risks posed by heavy metals through vegetable consumption. Clear differences were found in the concentrations of heavy metals in edible parts of the different vegetables. The concentrations of heavy metals decreased in the sequence as leafy vegetables > stalk vegetables/root vegetables/solanaceous vegetables > legume vegetables/melon vegetables. The ability of leafy vegetables to uptake and accumulate heavy metals was the highest, and that of melon vegetables was the lowest. This indicated that the low accumulators (melon vegetables) were suitable for being planted on contaminated soil, while the high accumulators (leafy vegetables) were unsuitable. In Shizhuyuan area, China, the total THQ values of adults and children through consumption of vegetables were 4.12 and 5.41, respectively, suggesting that the residents may be facing health risks due to vegetable consumption, and that children were vulnerable to the adverse effects of heavy metal ingestion.
Keywords: heavy metal, accumulation, health risk, vegetable, target hazard quotient (THQ)
1. Introduction
Heavy metal elements, such as lead (Pb), cadmium (Cd), arsenic (As), etc. , have toxic effects on human health. Toxic metals can accumulate persistently in the body over a lifetime. Pb can adversely influence the intelligence development of children, cause excessive lead in blood, and induce hypertension, nephropathy and cardiovascular disease [ 1 , 2 , 3 ]. Chronic Cd exposure can cause acute toxicity to the liver and lungs, induce nephrotoxicity and osteotoxicity, and impair function of the immune system [ 4 , 5 , 6 ]. The element As is a metalloid and is associated with angiosarcoma and skin cancer [ 7 , 8 ]. Other metal elements such as copper (Cu) and zinc (Zn) are important nutrients for humans, but excessive ingestion can also have adverse effects on human health [ 9 ]. For example, a Cu surplus can cause acute stomach and intestine aches, and liver damage [ 9 , 10 , 11 ], and Zn can reduce immune function and levels of high-density lipoproteins [ 11 , 12 ]. Compared with inhalation of soil particles, drinking water, and dermal contact, food consumption has been identified as the major pathway for human exposure to toxic metals [ 13 , 14 ].
Vegetables are important edible crops and are an essential part of the human diet. They are rich in nutrients required for human health, and are an important source of carbohydrates, vitamins, minerals, and fibers [ 15 , 16 ]. Heavy metals can be readily taken up by vegetable roots, and can be accumulated at high levels in the edible parts of vegetables, even heavy metal in soil at low levels [ 16 , 17 ]. In many countries and regions, vegetables are exposed to heavy metals by various means, thus vegetable consumption can cause adverse health effects. In Huludao City, China, the ranges of Pb and Cd concentrations in vegetables are 0.003–0.624 mg/kg and 0.003–0.195 mg/kg (fresh weight), respectively, and the maximum concentrations of Pb and Cd all exceed the recommended values (GB 2762-2005) [ 18 ]. Hu et al. [ 15 ] reported that 16%, 26%, and 0.56% of market vegetables in Hong Kong were contaminated by Pb, Cd, and Cr, respectively. Rahman et al. [ 9 ] reported that some Australian and Bangladeshi vegetables contained Cd concentrations higher than the Australian standard maximum limit (0.1 mg/kg). Therefore, vegetable consumption is considered to be one of the major sources of heavy metal intake for humans, and elevated levels of heavy metal in edible parts of vegetables can affect human health.
Vegetable species differ widely in their ability to take up and accumulate heavy metals, even among cultivars and varieties within the same species [ 19 , 20 ]. Alexander et al. [ 13 ] reported that Pb significantly accumulated in lettuce and onion, while Cd accumulated to the greatest extents in spinach and lettuce. Yang et al. [ 16 ] found that Chinese leek, pak choi, and carrot had higher Cd concentrations in their edible parts than radish, cucumber, and tomato. Säumel et al. [ 20 ] reported that Zn concentrations in green beans, tomato, potato, kahlrabi, and carrots were significantly lower than the concentrations in leafy vegetables. Cd accumulation in vegetable species decreased in the order of leafy vegetables > solanaceous vegetables > root vegetables > allimus vegetables > melon vegetables > legumes vegetables [ 21 ].
There are elevated levels of heavy metal in soils in many areas of the world, especially in developing countries and regions. In some mining and smelting areas in China, although the agriculture soils are contaminated with heavy metals, the farmers cannot afford to leave farmland fallow for remediation because the demand and pressure to produce foodstuffs and vegetables are so high. The selection and breeding of crop and vegetable species or cultivars that have low heavy metal accumulation, without having to leave the farmland fallow, seems to be a suitable method to reduce the adverse health effects of heavy metals [ 21 , 22 , 23 ]. There have been some studies that have focused on Cd contaminated soil and the selection of vegetable species or cultivars with low heavy metal accumulation [ 16 , 21 , 24 ]. However, very few studies have investigated multiple heavy metals (Pb, Cd, Cu, Zn, and As) in soils. Therefore, in this study, field experiments were carried out on Pb, Cd, Cu, Zn, and As contaminated farmland near Shizhuyuan Mine Zone in Chenzhou City, southern Hunan Province, China, where 22 vegetable species (six vegetable types) were used in the trials. The main purposes of this study were to investigate the concentrations and accumulation of Pb, Cd, Cu, Zn, and As in the edible parts of different vegetable species, and to assess the health risks of vegetable consumption on residents (adults and children).
2. Materials and Methods
2.1. experimental site and soil.
The climate of Shizhuyuan mine area in Suxian District of Chenzhou City is a continental middle subtropical monsoon humid climate zone, with a mean annual temperature in the range 15.6 °C –18.3 °C, a mean annual precipitation in the range 1400–1700 mm, and a frost-free period of about 295 days. The farmland under study (25°52.08′ N, 113°08.98′ E) is located near the East River of Shizhuyuan mine area. In 1985, a tailing dam was burst by a flood due to extreme rainstorms, causing the farmland near the East River to be flooded and covered with toxic tailings [ 25 , 26 ]. The tailings and contaminated top soils were removed by the government immediately after the accident, but there are still some farmlands contaminated with heavy metals that continue to produce foodstuffs and vegetables, which are eaten by the farmers and their families or are taken to the market for sale to urban residents. The farmland soil is contaminated with multiple metals (Pb, Cd, Cu, Zn, and As) in this study, and the basic properties of the farmland soil are given in Table 1 .
Basic properties of the farmland soil under study (d.w.).
a Environment quality standard for soils of China (GB 15618-1995).
2.2. Field Experiments
A total of 22 vegetable species are in common cultivation in Hunan Province, including three species of root vegetables, two species of stalk vegetables, three species of solanaceous vegetables, four species of melon vegetables, eight species of leafy vegetables, and two species of legume vegetables, and these were cultivated on the experimental farmland in the spring and autumn seasons ( Table 2 ). A rectangular experimental plot area of 8 m 2 (4 m × 2 m) was established for each vegetable in the farmland. Three replications were conducted for each vegetable. All of the plots were arranged in a randomized complete block design ( n = 66).
Vegetable species used in the experiments.
At harvest season (summer and winter, 2011), vegetables were collected at different maturation times. Five vegetable plants from each plot were collected and mixed to obtain a sample. Rhizosphere soil of each vegetable plant was separated from the roots by gently shaking the soil attached to the roots. All samples were stored in polyethylene bags for transport at a constant temperature of 4 °C. The vegetable samples were then cleaned using deionized water to remove dust and soil. The edible parts of the vegetables were separated from the plants, dried in an oven at 105 °C for 30 min, then dried in an oven at 70 °C until the weight of the sample remained constant. All samples were ground to fine powder by using a stainless steel grinder (RT-02B, China), and passed through a 100-mesh sieve, then kept in clean polyethylene container for further analysis. The soils were air-dried at room temperature, ground to fine powder by using an agate mortar and pestle, and passed through a 10-mesh sieve, then kept in clean polyethylene containers before analysis.
2.3. Target Hazard Quotient (THQ) Method
The potential health risks of heavy metal consumption through vegetables were assessed based on the target hazard quotient (THQ) method, which was described in detail by the United States Environmental Protection Agency [ 27 , 28 , 29 ]. The THQ is given by the following equation:
where E F is the exposure frequency (350 days/year); E D is the exposure duration (70 years, equivalent to the average lifetime of the Chinese population); F IR is the food ingestion rate (vegetable consumption values for adults and children are 301.0 and 231.5 g/person/day, respectively) [ 29 ]; C is the metal concentration in the edible parts of vegetables (mg/kg); R FD is the oral reference dose (Pb, Cd, Cu, Zn, and As values were 0.0035, 0.001, 0.040, 0.300, and 0.050 mg/kg/day, respectively) [ 30 , 31 , 32 ]; W AB is the average body weight (55.9 kg for adults and 32.7 kg for children) [ 18 , 29 ]; and T A is the average exposure time for non-carcinogens ( E D × 365 days/year). If the THQ value is greater than 1, the exposure is likely to cause obvious adverse effects.
The total THQ (TTHQ) of heavy metals for vegetables is given by the following equation [ 18 , 27 ]:
2.4. Chemical Analysis and Quality Control
Soil pH was measured in 1:2.5 of soil:water suspension using a glass electrode (PHS-3C, LEICI, Guangzhou, China). Soil organic matter (OM) content was determined by oxidation with potassium dichromate and colorimetric determination [ 33 ]. Cation exchange capacity (CEC) was determined using the ammonium acetate method after washing with alcohol [ 34 ]. The total concentrations of Pb, Cd, Cu, and Zn in soil samples were acid-digested with HCl/HNO 3 /HClO 4 , and the edible parts of the vegetables were digested by means of the dry ashing method [ 35 ], and determined using inductively coupled plasma optical emission spectrometry (ICP-OES 6300, Thermo Fisher Scientific, Waltham, MA, USA). The total As concentrations in soil samples were aqua-regia digested, and for the edible parts of the vegetables, a dry ashing method was used [ 36 ], and quantification of As by atomic fluorescence spectrometer was conducted (AFS-8220, Titan Instruments, Beijing, China). Certified reference materials for soil (GBW (E) 070009, geochemical certified reference soil of China) and certified reference materials for rice (GBW 10045, certified reference rice of China) were also analyzed with the samples. The standard reference materials were analyzed with the samples during the course of analysis. The mean recoveries of the standard reference materials soil were 95%, 92%, 105%, 92%, and 90% for Pb, Cd, Cu, Zn, and As, respectively, and the mean recoveries of standard reference rice of these metals were 92%, 94%, 98%, 109%, and 88%, respectively.
2.5. Statistical Analysis
A one-way ANOVA was used to evaluate the differences among vegetable species. Prior to ANOVA, the homogeneities of the variances were verified using Levene’s test. Duncan’s test was used to detect the significant differences between the means of different vegetable classifications. The criterion for significance in the procedures was set at p < 0.05 (significant). All data were presented as arithmetic means with standard error attached. All statistical analyses were conducted using the software Excel 2010 and SPSS Version19.0 (IBM Corporation, New York, NY, USA), and all figures were produced using Origin Version 8.5 software (OriginLab Corporation, Northampton, MA, USA).
3.1. Heavy Metal Concentrations in Vegetable Edible Parts
The concentrations of the heavy metals in the edible parts of the 22 species vegetables are presented in Table 3 . As can be seen, clear differences were found in the concentrations of the heavy metals. The observed ranges in the concentrations of Pb, Cd, Cu, Zn, and As in the edible parts were 0.004–2.361 mg/kg, 0.002–2.918 mg/kg, 0.155–3.125 mg/kg, 1.151–54.65 mg/kg, and 0.014–1.780 mg/kg, respectively, with mean concentrations of 0.383, 0.161, 0.810, 10.16, and 0.207 mg/kg, respectively. In all 22 species of vegetables, the Pb concentrations in the edible parts of 15 vegetable species, the Cd concentrations in the edible parts of eight vegetable species, and the As concentrations in the edible parts of four species vegetables were higher than the tolerance limit of contaminants in foods as set by the China National Standards (GB 2762-2012). The lowest concentrations of Pb, Cd, and As in the edible parts of 22 species vegetables were in cucumber ( Cucumis sativus L.), towel gourd ( Lu ff a cylindrica L.), and tomato ( Lycopersicum esculentum Mill.), while the highest concentrations were in edible amaranth ( Amaranthus tricolor L.). In addition, the coefficients of variation of Pb, Cd, Zn, and As concentrations were all greater than 1.0; the coefficient of variation for Cu was 0.85. The difference of Pb, Cd, and As concentrations between the highest and the lowest of the 22 vegetable species reached several hundred times, and even reached a thousand times, while the highest concentrations of Cu and Zn of 22 vegetable species were just a few times the lowest. These results indicated that clear differences in heavy metal (Pb, Cd, Cu, Zn, and As) bioaccumulation existed among the 22 species of vegetables.
Concentrations of heavy metals in vegetable edible parts (mg/kg, f.w.). Data show mean ± standard error of three replicates.
a The tolerance limit of contaminants in foods in the China National Standards (GB 2762-2012).
3.2. Heavy Metal Concentrations in Six Different Vegetable Types
The heavy metal concentrations in the edible parts of the six different vegetable types are shown in Figure 1 . Obvious differences were found in the concentrations of Pb, Cd, Cu, Zn, and As in the edible parts of the six vegetable types. The concentrations in the edible parts of the six vegetable types decreased in the order of leafy vegetables > stalk vegetables > root vegetables > solanaceous vegetables > legume vegetables > melon vegetables for Pb and Cd, legume vegetables > leafy vegetables > solanaceous vegetables > root vegetables > melon vegetables > stalk vegetables for Cu, leafy vegetables > stalk vegetables > legume vegetables > root vegetables > solanaceous vegetables > melon vegetables for Zn, and leafy vegetables > stalk vegetables > root vegetables > melon vegetables > legume vegetables > solanaceous vegetables for As. Consequently, the concentrations of Pb, Cd, Zn, and As in leafy vegetables were significantly higher than the other five vegetable types; however, this was not the case for Cu.
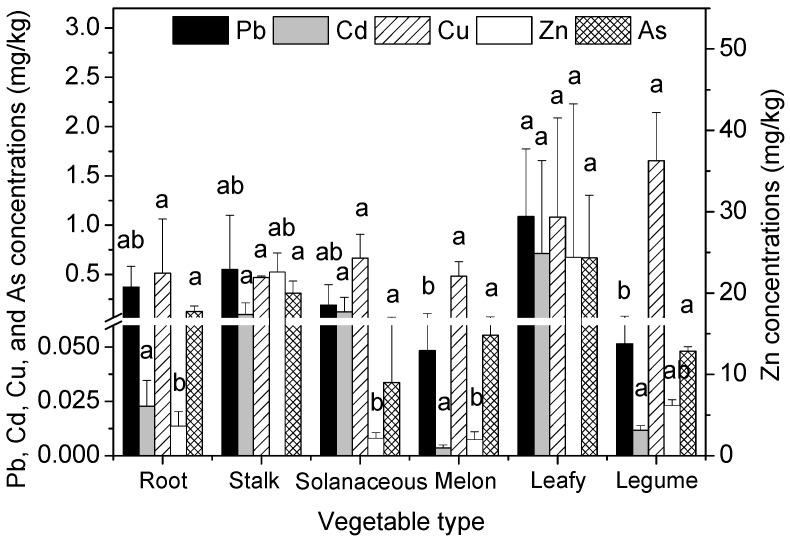
Heavy metal concentrations in the edible parts of six vegetable types (mg/kg, f.w.). Error bars indicate standard error of three replicates. The different letters indicate significant difference at p < 0.05 (Duncan’s test).
3.3. Bioaccumulation Factors (BAFs) of Heavy Metals in Six Vegetable Types
Bioaccumulation factors (BAFs), defined as the ratio of the metal concentrations in the edible parts of the vegetable to the metal concentrations in the soil (BAF= C plant /C soil ), can be used to estimate the ability of vegetables to accumulate metals in their edible parts. Significant differences were found in the BAFs of heavy metals in the edible parts of the six vegetable types ( Figure 2 ). The order of the heavy metal (Pb, Cd, Cu, Zn, and As) BAFs was similar to the order of the heavy metal concentrations ( Figure 1 ). The BAFs of Pb, Cd, Cu, Zn, and As in the edible parts of leafy vegetables were higher than for the other five vegetable types. Significant differences ( p < 0.05) were found in the BAFs of Pb, Cd, and Zn in the edible parts of leafy vegetables and solanaceous vegetables, melon vegetables, and legume vegetables, respectively. The BAFs of all vegetable types were less than 1.0, except for leafy vegetables (1.11). Additionally, the BAFs of Cd, Cu, and Zn were higher than the BAFs of Pb and As ( Figure 2 ). These results indicated that the ability for metal accumulation in the edible parts of leafy vegetables was higher than that for the other five vegetable types, and the ability of Cd, Cu, and Zn accumulation in the edible parts was much greater than that for Pb and As.
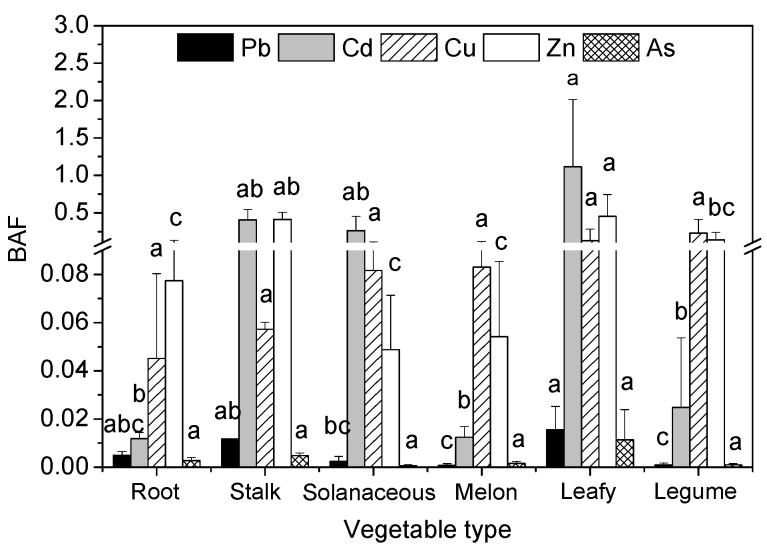
Bioaccumulation factors (BAFs) of heavy metals in six vegetable types. Error bars indicate standard error of three replicates. The different letters indicate significant difference at p < 0.05 (Duncan’s test).
3.4. Health Risk Assessment of Vegetable Consumption
Chronic low-level intake of toxic metal elements has a negative effect on human health, and the detrimental impact becomes apparent after several years of exposure [ 30 , 37 , 38 ]. The THQ method was used to assess the potential health risks of heavy metal accumulation through vegetable consumption in this study. Vegetable consumption values for adults and children in China are 301.0 and 231.5 g/person/day, respectively [ 29 ]. In the consumption habits of local residents, the leafy vegetable consumption accounted for 65% of total consumption of vegetables, followed by solanaceous and melon vegetables, each of them accounted for 10%, and then each of stalk, root, and legumes vegetables accounted for 5%. The THQ values of Pb, Cd, Cu, Zn, and As due to vegetable consumption for residents (adults and children) of the study area are listed in Table 4 . The THQ values of Pb, Cd, Cu, Zn, and As through consumption of leafy vegetables for residents were higher than for the other five vegetable types. For leafy vegetables, the Pb THQ values of adults and children were 1.04 and 1.37, respectively, and Cd THQ values were 2.39 for adults, 3.15 for children, while there were no THQ values for Cu, Zn, and As greater than 1.0 through the consumption of the six vegetable types alone. These results suggested that the potential health risks of heavy metals through consumption of leafy vegetables were the highest for all vegetable types. The THQ values of Pb, Cd, Cu, Zn, and As through vegetable consumption for children were higher than the values for adults in six vegetable types in the present study, indicating that the potential health risks facing for children were greater than for adults. In addition, the total diet THQ of each metal (TDHQ) values of heavy metals decreased in the order Cd > Pb > Zn > Cu > As ( Table 4 ). This suggested that for toxic heavy metals, the potential health risks of Cd and Pb through vegetable consumption were higher than for As.
Heavy metal target hazard quotient (THQ) values due to consumption of six vegetable types.
a The total diet THQ of each metal (total diet, i.e. , the sum of root, stalk, solanaceous, melon, leafy, and legume).
It has been reported that exposure to two or more pollutants may result in additive and/or interactive adverse effects [ 29 ]. Therefore, it is hard to assess the potential health risks of multiple metals using each individual THQ value for the heavy metals. Furthermore, the total THQ (TTHQ) of heavy metals is the sum of the individual heavy metal (Pb, Cd, Cu, Zn, and As) THQ values for the six vegetable types, and the values are shown in Figure 3 . The TTHQ values for adults and children through vegetable consumption were 4.12 and 5.41, respectively. This result indicated that the residents of the Shizhuyuan area may be facing health risk, and the potential health risks for children were greater than for adults. The relative contributions of Pb, Cd, Cu, Zn, and As to the TTHQ are 27.9%, 60.5%, 3.0%, 7.5%, and 1.2%, respectively. Consequently, Pb and Cd were the main elements contributing to the potential health risks of vegetable consumption for residents in the study area.
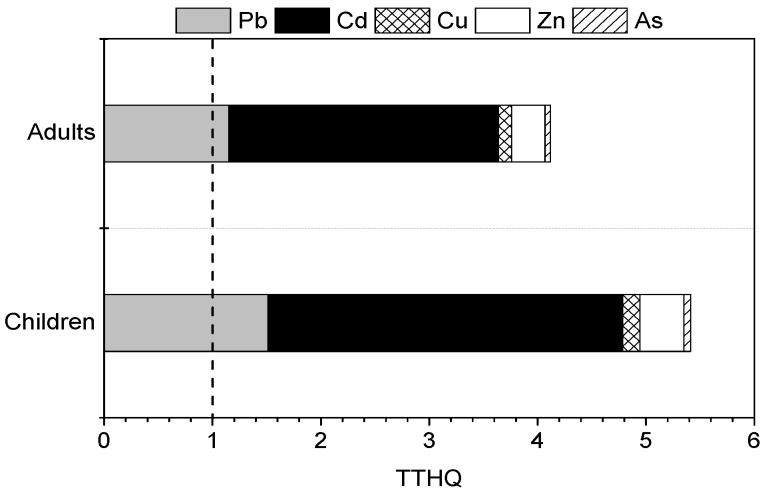
TTHQ values due to consumption of vegetables.
4. Discussion
Vegetable species differ widely in their ability to take up and accumulate heavy metals, even among cultivars and varieties within the same species [ 19 , 20 ]. It has been reported that Cd uptake and accumulation in leafy vegetables are greater than in non-leafy vegetables [ 16 , 21 ]. In this study, significant differences were found in the concentrations of heavy metals in the edible parts of different vegetable types; the concentrations decreased in the order of leafy vegetables > stalk vegetables/root vegetables/solanaceous vegetables > legume vegetables/melon vegetables ( Table 3 ). In addition, the ability for heavy metal uptake and accumulation of leafy vegetables was higher than for the other vegetable types, and the ability of melon vegetables was the lowest of all vegetable types studied ( Figure 2 ). Edible amaranth, spinach, and caraway had higher concentrations and BAFs of Pb, Cd, Zn, and As ( Table 3 , Figure 2 ), and were classed as “high accumulators” [ 13 , 19 , 21 ]. Lower concentrations and BAFs of Pb, Cd, Zn, and As were found in bitter gourd, towel gourd, cucumber, and pumpkin ( Table 3 , Figure 2 ), which were classed as “low accumulators” [ 13 , 21 ]. This suggested that the low accumulators were suitable for being planted on contaminated soil, while the high accumulators were unsuitable [ 13 , 19 ]. The elevated concentration levels of heavy metals and the strong ability for heavy metal accumulation in leafy vegetables were possibly due to the leaves being the main parts of the vegetables used for photosynthesis, because higher metal mass flowed to the leaves due to strong transpiration [ 39 , 40 , 41 ]; the leaves were also easily exposed to contaminated soil because leafy vegetables were generally dwarfish plants with leaves closer to the ground than the other types of vegetables. Furthermore, atmospheric heavy metal deposition might be one of the reasons for elevated metal concentrations in leafy vegetables in mining and smelting areas [ 37 , 42 ].
Obvious differences in accumulation of heavy metals (Pb, Cd, Cu, Zn, and As) were found in the same vegetable species. The Cd, Cu, and Zn concentrations in soil were lower than Pb and As, while the Cu and Zn concentrations in edible parts were significantly higher than Pb, Cd, and As in all studied vegetables ( Table 3 ). These probably were because Cu and Zn were the essential elements for vegetables growth [ 9 ], and were readily accumulated in roots and transported to aerial part [ 43 ]; while Pb, Cd, and As were the toxic elements and were not required for vegetables growth, they were stored in roots, and transport to aerial parts of the plant was limited [ 16 , 44 ]. Although Cd concentration in soil was lower than Pb and As concentrations ( Table 1 ), the Cd concentrations in edible parts of vegetables were the same levels as Pb and As ( Table 3 ), and the BAF values of Cd in edible parts were significantly higher than those of Pb and As ( Figure 2 ). These results suggested that Cd had greater accumulation ability in edible parts of vegetables than Pb and As, and the roots were more likely to transport Cd to aerial part [ 19 , 43 ]. Furthermore, the Pb concentrations in edible parts were found to be highly significant positively ( p < 0.01) correlated with the concentrations of Cd, Zn, and As, and the correlation coefficients ( r ) were 0.872, 0.870, and 0.754 ( n = 22, r 0.01 = 0.53), respectively; the Cd concentrations were highly significant positively ( p < 0.01) correlated with the concentrations of Zn and As, and the correlation coefficients ( r ) were 0.806 and 0.784, respectively; the As concentrations were highly significant positively ( p < 0.01) correlated with the Zn concentrations ( r = 0.649). It seemed that the absorption, transport, and accumulation of Pb, Cd, As in edible parts might have a relationship with those of Zn.
Generally, Cu and Zn, which are important nutrients for humans, are considered a much lower health risk to humans than Pb, Cd, and As [ 13 ]. Poor health can be caused by a lack of these required elements [ 14 ], but excessive ingestion can also have adverse effects on human health [ 9 , 15 ]. At present, there are several methods to estimate the potential health risks of pollutants for carcinogenic and non-carcinogenic effects [ 18 , 27 , 29 , 32 ]. Non-cancer risk assessment is typically based on the THQ method, which is a ratio of the determined dose of a pollutant to the reference oral dose ( R FD ) [ 27 , 28 , 29 ]. The THQ values are associated with many factors, including intake of pollutants, exposure time, body weight, and reference oral dose of pollutants. The significantly different THQ values for adults and children were due to the differences in the intake of metals, exposure time, and body weight between adults and children in this study ( Table 4 ). Obvious differences had been found in THQ values in males and females through vegetable consumption in Banat Country, Romania, and THQ values for females were higher than those for males [ 11 ]. This indicated that the potential health risks for children were higher than those for adults, and that the potential health risks for females were higher than those for males. The TTHQ values of residents in the current study area through vegetable consumption exceeded 1.0 ( Figure 3 ), suggesting that the residents may be facing health risk. Additionally, for special populations, such as those with a weak constitution, those that were sensitive, and women that were pregnant, the potential health risks of heavy metal accumulation through vegetable consumption were likely to be higher than for the normal population. However, vegetable consumption was just one part of food consumption. In addition to vegetable consumption, rice [ 18 , 31 ], meat [ 18 , 30 , 45 ], fish [ 29 , 32 ], and tobacco [ 46 ] consumption also led to ingestion of large amounts of heavy metals. For the residents of Shizhuyuan mining area, food consumption, inhalation of soil particles, drinking water, and dermal contact were the important pathways for human exposure to toxic metals [ 13 , 14 ]. Consequently, the potential health risks for residents were actually higher than the results from this study. Fortunately, the government has realized the adverse effects and the significant health risks posed by heavy metals in recent years, and some remediation measures have already been undertaken on contaminated soils to reduce health risks.
5. Conclusions
The present study cultivated 22 species vegetables (six vegetable types) on heavy metal (Pb, Cd, Cu, Zn, and As) contaminated farmland. The concentrations of heavy metals in the edible parts of the vegetables decreased in the order of leafy vegetables > stalk vegetables/root vegetables/solanaceous vegetables > legume vegetables/melon vegetables. The ability for heavy metal uptake and accumulation of leafy vegetables was the highest, and that of melon vegetables was the lowest. These results indicated that the low accumulators (melon vegetables) should be suitable for being planted on contaminated soil, while the high accumulators (leafy vegetables) are unsuitable.
The TTHQ values of adults and children through vegetable consumption were 4.12 and 5.41, respectively, suggesting that the residents of Shizhuyuan area may be facing health risks due to vegetable consumption, and that children were particularly vulnerable to the adverse effects of ingestion of heavy metals. Pb and Cd were the main elements contributing to potential health risks of vegetable consumption for residents in the study area. The potential health risks of heavy metals through other exposure pathways should be the subject of future study.
Acknowledgments
This study received financial support from the National Natural Science Foundation of China (No. 41501344), the Science Research Project of the Agriculture Department in Hunan Province (No. XNYL 2015-112-2), and the Science Research Project of the Education Department in Hunan Province (No. 15K148).
Author Contributions
Hang Zhou and Xin Zhou wrote the paper; Hang Zhou, Bo-Han Liao, and Pei-Qin Peng designed the experiments; Hang Zhou, Wen-Tao Yang and Wen-Lei Wang analyzed the data and produced the figures; Jiao-Feng Gu, Li Liu, Jia-Ling Zou, and Tao Tian implemented the experiments, and collected and analyzed all of the soil and vegetable samples.
Conflicts of Interest
The authors declare no conflict of interest.
- 1. Ekong E.B., Jaar B.G., Weaver V.M. Lead-related nephrotoxicity: A review of the epidemiologic evidence. Kidney Int. 2006;70:2074–2084. doi: 10.1038/sj.ki.5001809. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Goyer R.A. Lead Toxicity: Current Concerns. Environ. Health Persp. 1993;100:177–187. doi: 10.1289/ehp.93100177. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Navas-Acien A., Guallar E., Silbergeld E.K., Rothenberg S.J. Lead exposure and cardiovascular disease: A systematic review. Environ. Health Perspect. 2007;115:472–482. doi: 10.1289/ehp.9785. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Klaassen C.D., Liu J., Choudhuri S. Metallothionein: An intracellular protein to protect against cadmium toxicity. Annu. Rev. Pharmacol. 1999;39:267–294. doi: 10.1146/annurev.pharmtox.39.1.267. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Klaassen C.D., Liu J., Diwan B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharm. 2009;238:215–220. doi: 10.1016/j.taap.2009.03.026. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Patrick L. Toxic metals and antioxidants: Part II. The role of antioxidants in arsenic and cadmium toxicity. Altern. Med. Rev. 2003;8:106–128. [ PubMed ] [ Google Scholar ]
- 7. Chiou H.Y., Hsueh Y.M., Liaw K.F., Horng S.F., Chiang M.H., Pu Y.S., Lin J.S., Huang C.H., Chen C.J. Incidence of internal cancers and ingested inorganic arsenic: A seven-year follow-up study in Taiwan. Cancer Res. 1995;55:1296–1300. [ PubMed ] [ Google Scholar ]
- 8. Hartley W., Lepp N.W. Remediation of arsenic contaminated soils by iron-oxide application, evaluated in terms of plant productivity, arsenic and phytotoxic metal uptake. Sci. Total Environ. 2008;390:35–44. doi: 10.1016/j.scitotenv.2007.09.021. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Rahman M.A., Rahman M.M., Reichman S.M., Lim R.P., Naidu R. Heavy metals in Australian grown and imported rice and vegetables on sale in Australia: Health hazard. Ecotoxicol. Environ. Saf. 2014;100:53–60. doi: 10.1016/j.ecoenv.2013.11.024. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Gaetke L.M., Chow C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189:147–163. doi: 10.1016/S0300-483X(03)00159-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Harmanescu M., Alda L.M., Bordean D.M., Gogoasa L., Gergen L. Heavy metals health risk assessment for population via consumption of vegetables grown in old mining area, a case study: Banat County, Romania. Chem. Cent. J. 2011;5:64–73. doi: 10.1186/1752-153X-5-64. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Chandra R.K. Excessive intake of zinc impairs immune responses. JAMA. 1984;252:1443–1446. doi: 10.1001/jama.1984.03350110043027. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Alexander P.D., Alloway B.J., Dourado A.M. Genotypic variations in the accumulation of Cd, Cu, Pb and Zn exhibited by six commonly grown vegetables. Environ. Pollut. 2006;144:736–745. doi: 10.1016/j.envpol.2006.03.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Zhu F., Fan W., Wang X., Qu L., Yao S. Health risk assessment of eight heavy metals in nine varieties of edible vegetable oils consumed in China. Food Chem. Toxicol. 2011;49:3081–3085. doi: 10.1016/j.fct.2011.09.019. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Hu J., Wu F., Wu S., Cao Z., Lin X., Wong M.H. Bioaccessibility, dietary exposure and human risk assessment of heavy metals from market vegetables in Hong Kong revealed with an in vitro gastrointestinal model. Chemosphere. 2013;91:455–461. doi: 10.1016/j.chemosphere.2012.11.066. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Yang Y., Zhang F.S., Li H.F., Jiang R.F. Accumulation of cadmium in the edible parts of six vegetable species grown in Cd-contaminated soils. J. Environ. Manag. 2009;90:1117–1122. doi: 10.1016/j.jenvman.2008.05.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Jolly Y.N., Islam A., Akbar S. Transfer of metals from soil to vegetables and possible health risk assessment. SpringerPlus. 2013;2:385–391. doi: 10.1186/2193-1801-2-385. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Zheng N., Wang Q., Zhang X., Zheng D., Zhang Z., Zhang S. Population health risk due to dietary intake of heavy metals in the industrial area of Huludao city, China. Sci. Total Environ. 2007;387:96–104. doi: 10.1016/j.scitotenv.2007.07.044. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Zhu Y., Yu H., Wang J., Fang W., Yuan J., Yang Z. Heavy metal accumulations of 24 asparagus bean cultivars grown in soil contaminated with Cd alone and with multiple metals (Cd, Pb, and Zn) J. Agric. Food Chem. 2007;55:1045–1052. doi: 10.1021/jf062971p. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Säumel I., Kotsyuk I., Hölscher M., Lenkereit C., Weber F., Kowarik I. How healthy is urban horticulture in high traffic areas? Trace metal concentrations in vegetable crops from plantings within inner city neighbourhoods in Berlin, Germany. Environ. Pollut. 2012;165:124–132. doi: 10.1016/j.envpol.2012.02.019. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Yang J., Guo H., Ma Y., Wang L., Wei D., Hua L. Genotypic variations in the accumulation of Cd exhibited by different vegetables. J. Environ. Sci. China. 2010;22:1246–1252. doi: 10.1016/S1001-0742(09)60245-X. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Ding C., Zhang T., Wang X., Zhou F., Yang Y., Yin Y. Effects of soil type and genotype on lead concentration in rootstalk vegetables and the selection of cultivars for food safety. J. Environ. Manag. 2013;122:8–14. doi: 10.1016/j.jenvman.2013.02.026. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Xin J., Huang B., Yang Z., Yuan J., Dai H., Qiu Q. Responses of different water spinach cultivars and their hybrid to Cd, Pb and Cd–Pb exposures. J. Hazard Mater. 2010;175:468–476. doi: 10.1016/j.jhazmat.2009.10.029. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Dunbar K.R., McLaughlin M.J., Reid R.J. The uptake and partitioning of cadmium in two cultivars of potato (Solanum tuberosum L.) J. Exp. Bot. 2003;54:349–354. doi: 10.1093/jxb/erg016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Lei M., Zhang Y., Khan S., Qin P., Liao B. Pollution, fractionation, and mobility of Pb, Cd, Cu, and Zn in garden and paddy soils from a Pb/Zn mining area. Environ. Monit. Assess. 2010;168:215–222. doi: 10.1007/s10661-009-1105-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Zeng Q.R., Sauve S., Allen H.E., Hendershot W.H. Recycling EDTA solutions used to remediate metal-polluted soils. Environ. Pollut. 2005;133:225–231. doi: 10.1016/j.envpol.2004.06.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Storelli M.M. Potential human health risks from metals (Hg, Cd, and Pb) and polychlorinated biphenyls (PCBs) via seafood consumption: Estimation of target hazard quotients (THQs) and toxic equivalents (TEQs) Food Chem. Toxicol. 2008;46:2782–2788. doi: 10.1016/j.fct.2008.05.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. State of Oregon Department of Environmental Quality . Risk-Based Concentration Table. United States Environmental Protection Agency; Philadelphia, CA, USA: 2000. [ Google Scholar ]
- 29. Wang X., Sato T., Xing B., Tao S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005;350:28–37. doi: 10.1016/j.scitotenv.2004.09.044. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Bortey-Sam N., Nakayama S.M.M., Ikenaka Y., Akoto O., Baidoo E., Yohannes Y.B., Mizukawa H., Ishizuka M. Human health risks from metals and metalloid via consumption of food animals near gold mines in Tarkwa, Ghana: Estimation of the daily intakes and target hazard quotients (THQs) Ecotoxicol. Environ. Saf. 2015;111:160–167. doi: 10.1016/j.ecoenv.2014.09.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Hang X., Wang H., Zhou J., Ma C., Du C., Chen X. Risk assessment of potentially toxic element pollution in soils and rice (Oryza sativa) in a typical area of the Yangtze River Delta. Environ. Pollut. 2009;157:2542–2549. doi: 10.1016/j.envpol.2009.03.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Iwegbue C.M.A. Metal concentrations in selected brands of canned fish in Nigeria: Estimation of dietary intakes and target hazard quotients. Environ. Monit. Assess. 2015;187:1–15. doi: 10.1007/s10661-014-4135-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Sparks D.L., Page A.L., Helmke P.A., Loeppert R.H., Soltanpour P.N., Tabatabai M.A., Johnston C.T., Sumner M.E. Methods of Soil Analysis, Part 3–Chemical Methods. Soil Science Society of America Inc.; Madison, WI, USA: 1996. pp. 961–1010. [ Google Scholar ]
- 34. Kahr G., Madsen F.T. Determination of the cation exchange capacity and the surface area of bentonite, illite and kaolinite by methylene blue adsorption. Appl. Clay Sci. 1995;9:327–336. doi: 10.1016/0169-1317(94)00028-O. [ DOI ] [ Google Scholar ]
- 35. Hseu Z.Y. Evaluating heavy metal contents in nine composts using four digestion methods. Bioresour. Technol. 2004;95:53–59. doi: 10.1016/j.biortech.2004.02.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Tam G.K., Lacroix G. Dry ashing, hydride generation atomic absorption spectrometric determination of arsenic and selenium in foods. J. Assoc. Off. Anal. Chem. 1982;65:647–650. [ PubMed ] [ Google Scholar ]
- 37. Huang S.S., Liao Q.L., Hua M., Wu X.M., Bi K.S., Yan C.Y., Chen B., Zhang X.Y. Survey of heavy metal pollution and assessment of agricultural soil in Yangzhong district, Jiangsu Province, China. Chemosphere. 2007;67:2148–2155. doi: 10.1016/j.chemosphere.2006.12.043. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Liu H., Probst A., Liao B. Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China) Sci. Total Environ. 2005;339:153–166. doi: 10.1016/j.scitotenv.2004.07.030. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Marchiol L., Assolari S., Sacco P., Zerbi G. Phytoextraction of heavy metals by canola (Brassica napus) and radish (Raphanus sativus) grown on multicontaminated soil. Environ. Pollut. 2004;132:21–27. doi: 10.1016/j.envpol.2004.04.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Perfus-Barbeoch L., Leonhardt N., Vavasseur A., Forestier C. Heavy metal toxicity: Cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 2002;32:539–548. doi: 10.1046/j.1365-313X.2002.01442.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Zhou H., Zeng M., Zhou X., Liao B., Liu J., Lei M., Zhong Q., Zeng H. Assessment of heavy metal contamination and bioaccumulation in soybean plants from mining and smelting areas of southern Hunan province, China. Environ. Toxicol. Chem. 2013;32:2719–2727. doi: 10.1002/etc.2389. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Sharma R.K., Agrawal M., Marshall F.M. Heavy metal (Cu, Zn, Cd and Pb) contamination of vegetables in urban India: A case study in Varanasi. Environ. Pollut. 2008;154:254–263. doi: 10.1016/j.envpol.2007.10.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Zhou H., Zeng M., Zhou X., Liao B.H., Peng P.Q., Hu M., Zhu W., Wu Y.J., Zou Z.J. Heavy metal translocation and accumulation in iron plaques and plant tissues for 32 hybrid rice (Oryza sativa L.) cultivars. Plant Soil. 2015;386:317–329. doi: 10.1007/s11104-014-2268-5. [ DOI ] [ Google Scholar ]
- 44. Liu W.X., Shen L.F., Liu J.W., Wang Y.W., Li S.R. Uptake of toxic heavy metals by rice (Oryza sativa L.) cultivated in the agricultural soil near Zhengzhou City, People’s Republic of China. Bull. Environ. Contam. Toxicol. 2007;79:209–213. doi: 10.1007/s00128-007-9164-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Barone G., Storelli A., Garofalo R., Busco V.P., Quaglia N.C., Centrone G., Storelli M.M. Assessment of mercury and cadmium via seafood consumption in Italy: Estimated dietary intake (EWI) and target hazard quotient (THQ) Food Addit. Contam. A. 2015;32:1277–1286. doi: 10.1080/19440049.2015.1055594. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Dong Z., Bank M.S., Spengler J.D. Assessing Metal Exposures in a Community near a Cement Plant in the Northeast US. Int. J. Environ. Res. Public Health. 2015;12:952–969. doi: 10.3390/ijerph120100952. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (769.0 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Health Risk of Heavy Metals Related to Consumption of Vegetables in Areas of Industrial Impact in the Republic of Kazakhstan—Case Study for Oskemen
Laura boluspayeva, monika jakubus, waldemar spychalski, akhan abzhalelov, yertas bitmanov.
- Author information
- Article notes
- Copyright and License information
Correspondence: [email protected]
Received 2022 Nov 24; Revised 2022 Dec 20; Accepted 2022 Dec 20; Collection date 2023 Jan.
Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( https://creativecommons.org/licenses/by/4.0/ ).
Among various heavy metal sources the metallurgic industry is the most threatening because emitted metals presented are the chemical forms in which metals are found in soil are more bioavailable and thus very easily are introduced into the environment and spread in both soils and plants. In this study such a situation is presented and the potential negative effect of emitted metals on soil and vegetables is estimated. Therefore, the following indicators were used: bioconcentration factors calculated for the total amount of metals (BCF) as well as daily intake of metal (DIM) and health risk index (HRI). Analyzed soils and vegetables originated from allotment gardens located at different distances from local industrial plants. The greatest amounts of metals in investigated materials (soils and plants) were found for the industrial zone and the lowest for samples representing the suburban zone. Among the analyzed metals Zn showed the highest (223.94–2645.13 mg·kg −1 for soils and 9.14–49.28 mg·kg −1 for plants), and Cd the lowest levels (1.77–15.2 mg·kg −1 for soils and 0.05–0.46 mg·kg −1 for plants). Regardless of the metal, the lowest BCF values were calculated for plants from the industrial zone and the highest from the urban site. Generally, BCF values calculated for vegetables were low and comparable for carrots, tomatoes, and cabbage. BCF values obtained for beetroots were higher in comparison to other vegetables. Regardless of plants, DIM values for Cd and Pb were low and comparable. DIM values for Cu and Zn were higher, but simultaneously strongly differentiated depending on the analyzed vegetables. A similar tendency was found in the case of HRI. The highest values were recorded for Cu and Zn in tomatoes. Regardless of the individual metals, the calculated values for DIM and HRI indices increased in the following sequence: beetroot < cabbage < carrot < tomato. The Zn and Cu contents in the studied types of vegetables do not exceed the maximum permissible levels recommended by WHO/FAO. In contrast, Pb concentrations were higher than the imposed standards in all the analyzed vegetable samples. On the basis of obtained DIM and HRI indices, consumption of vegetables cultivated in industrial areas should be restricted due to health risks related to heavy metals contained in plants.
Keywords: bioconcentration factors (BCF), daily intake of metal (DIM), health risk index (HRI), industrial and urban zones, allotment gardens
1. Introduction
According to the United States Environmental Protection Agency (EPA) compilation, seven heavy metals, Pb, Cr, Zn, Cu, Cd, Hg, Ni, and a metalloid such as As, are listed to be the most widespread heavy metals in the environment [ 1 ]. Heavy metals constitute a group of elements characterized by high density and high toxicity even at low concentrations in the environment, so taking under consideration metal toxicity to living organisms Zwolak et al. [ 2 ] proposed the following order: Hg > Cu > Zn > Ni > Pb > Cd > Cr > Sn > Fe > Mn > Al. The group of heavy metals is very differentiated because there are both very toxic, non-essential metals (Pb, Cd, Hg, Al) and essential micronutrients as Cu, Ni, and Zn. The latter metals play an important role in the metabolic pathways during plant growth and development when available in required concentrations, but not higher. Heavy metals continue to be released into the environment posing a threat to the health of people exposed to them both through inhalation and food and these hazardous pollutants originate from many sources, first of all, mining, smelting activity, steel and iron industry, chemical industry, and transport [ 3 , 4 , 5 ]. The emitted metals penetrate into environments where soil is their primary reservoir. Therefore, this environment component plays an essential role in global metal cycles. It is of particular importance in the case of agricultural and horticultural soils, where heavy metals may be transferred from the soil to the cultivated plants and accumulated in their tissues. Contamination through different food chains is the major pathway of heavy metal exposure for humans. Moreover, humans are the last link in the food chain so they are particularly exposed to the negative effects of heavy metals [ 4 , 6 , 7 ]. Thus, direct consumption of fruits and vegetables is mainly responsible for the potential contamination of human organisms. Moreover, it is now widely recognised that heavy metals taken up by plants constitute a predominant source of their accumulation in food [ 8 ]. In view of the above, special attention is focused on plants cultivated in close surroundings of metallurgic plants as well as mines and smelting works. Very often the concentration of heavy metals in plants is not assessed or controlled while at the same time posing a significant risk of contamination of the human diet, particularly, when inhabitants cultivate vegetables and other crop plants for their own needs in family allotment gardens. Family allotment gardens are very popular and play important functions both for cities and their inhabitants. Unfortunately, such gardens were established in relatively unattractive sites, near industrial plants, railways, and car transport routes [ 9 ]. As a consequence, the obtained plant products may be contaminated with heavy metals emitted from nearby sources. Consumption of such fruits or vegetables can greatly affect human health. Gupta et al. [ 4 ] indicated that dietary intake of metals through contaminated vegetables may cause various chronic diseases. Sandeep et al. [ 5 ] discussed this issue in detail, underlining human health implications of individual heavy metals. The negative impact of heavy metals contained in plant products on the human will directly depend on the quantity and quality of consumed products, in this case heavy metal amounts. Such a potential risk should be assessed in several areas, taking into account the effectiveness of metal bioconcentration in organs and tissues of consumed vegetables and the daily intake of metals through consumptions of various plant products. Based on the amount of metals in the soil and in plants the transfer of heavy metals from soil to plants should be assessed, with bioconcentration factors (BCF) being useful indices in this respect [ 10 , 11 ]. Furthermore, the probable human risk associated with heavy metals should also be evaluated. Various indices and parameters are used to assess the human health risk posed by heavy metals through vegetable consumption. Gupta et al. [ 4 ], Nag et al. [ 6 ] and Kumar et al. [ 12 ] listed such indices including the target, hazard quotient, daily dietary intake of metals, hazard index, daily intake of metals and health risk index. A literature review shows that the most popular indices are daily intake of metals (DIM) and health risk index (HRI) [ 9 , 11 , 13 , 14 , 15 , 16 ]. Daily intake of metals can help assess the relative phyto-availability of metals and is proportional to metal concentrations in the edible parts of vegetables as well as the consumed amounts of those vegetables. The health risk index underlines the significance of DIM in relation to the reference dose (RfD) of each metal. The value of HRI < 1 assumes no risk of a non-carcinogenic effect and the exposed population is said to be safe. HRI >1 is assumed to have potential significant non-carcinogenic effects.
In this study, the above indices were used for the first time to assess the potential threat to soil and plants posed by the metallurgical industry and its heavy metals emissions in the largest industrial city (Oskemen) in the Republic of Kazakhstan. The total area of the city is 230 km 2 and it is inhabited by 300,000 people. There are over 400 plants in the city, which together emit almost 70,000 Mg of hazardous substances, including mainly copper, zinc, lead, and cadmium compounds. The dominant reason for undertaking this research was to draw the attention of the local community, especially decision-makers, to the problem of the presence of heavy metals in the environment with its dangerous and direct impact on human health as a result of both cultivation and consumption of vegetables potentially loaded with metals.
Therefore, the present study is aimed: 1. to determine heavy metal amounts in soils and edible parts of selected vegetables: carrot, cabbage, tomato, and beetroot, 2. to assess the potential intensity of heavy metal concentrations in edible parts of vegetables as the bioconcentration factor (BCF), and 3. to investigate the potential health hazard associated with both daily intake of metals (DIM) and health risk index (HRI).
2. Materials and Methods
2.1. study area.
The study was conducted in four separate zones (I—central urban zone, II—suburban zone, III—industrial zone, IV—north-eastern industrial zone) in the city of Oskemen ( Figure 1 ). It is the largest industrial city in the northeast of the Republic of Kazakhstan, where the mining industry and metallurgy are very well developed. The applied division of the city into zones was dictated by the distribution of industry and the average population density. The authors took into account the intensity of the industrial load, the distance from emission sources and the number of residential buildings. The urban area (I zone) includes a significant part of the residential areas of the regional center. The suburban southern area (II zone) includes: a silk fabric factory, the Left Coast, Ablaketka, and residential areas in neighborhood of the Okemen hydroelectric power station. The northern industrial zone (III zone) is represented by the industrial sites of UKMC Kazzinc LLP, Ulba Metallurgical Plant JSC, AES JSC Oskemen CHPP and the territories surrounded to them. On the north-eastern industrial area (IV zone) the JSC Oskemen Titanium-magnesium plant and Sogrinsk thermal power station are located. According to WRB [ 17 ], the soils of the study area are primarily represented by Haplic Chernozems, Gleyic Chernozems and Luvic Chernozems. The soil samples were taken from a depth of 0–20 cm from allotment gardens located in four zones of the study area ( Figure 1 ). Each soil sample of approximately 0.5 kg collected in selected sites consisted of 15 individual punctures with a soil sampler. The collected samples were dried to an air-dry state, then ground in a porcelain mortar and sieved through a nylon sieve with a mesh size of 2 mm. According to Boluspaeva et al. [ 18 ], soils in the study area are characterized by a sandy silt or loam sand texture, which was shown by the percentage of clay ranging from 2.9 to 6.1% ( Table 1 ). The pH of the analysed soils ranged from neutral to basic, and its values expressed in pH units ranged from 6.42 to 7.70. The cation exchange capacity (CEC) was relatively high and reached 8.1–25.3 cmol (+)·kg −1 . The total carbon (TC) content ranged from 0.98 to 5.55%, but most often exceeded the value of 2% ( Table 1 ). The mean amount of total nitrogen (TN) was 0.20% in the range from 0.09 to 0.36%. The C:N values oscillated around 12:1 ( Table 1 ).
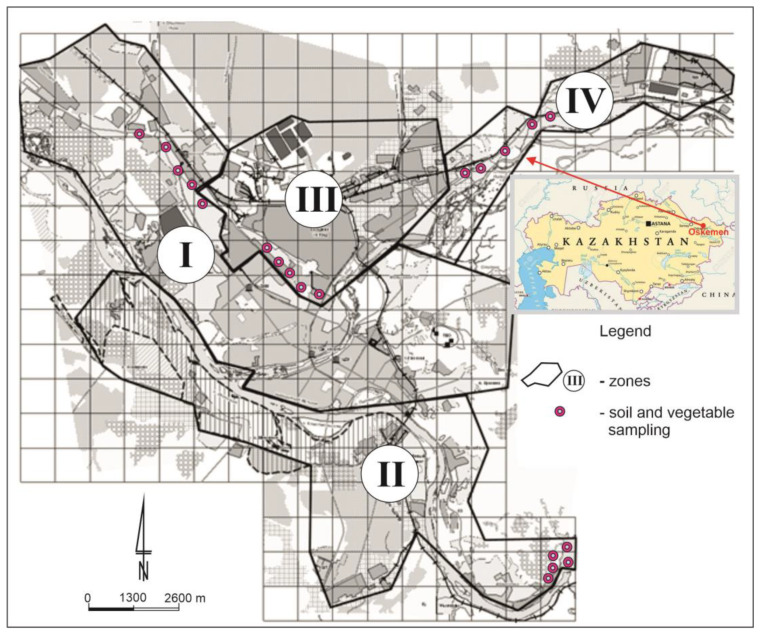
Study area with the location of zones and soil and vegetable sampling sites.
General characteristics of soils representing individual zones.
* I—central urban zone, II—suburban zone, III—industrial zone, IV—north-eastern industrial zone ** range of values; *** mean values ± SD.
The following vegetables, carrot, tomato, cabbage and red beetroot, were collected in the same locations as soil samples. All vegetable samples were washed with distilled water, crushed and dried at 60 °C. After drying they were homogenized in a mortar.
2.2. Methods
Soil texture was determined using a laser particle size analyzer Mastersizer 2000 with a Hydro MU dispersion unit (Malvern Panalytical, Malvern, UK). The chemical composition of the soils was analyzed in the <2 mm fraction. The soil pH was measured potentiometrically in 0.01 M CaCl 2 solution with a soil/extractant ratio of 1:5. Sorption capacity was determined in an extract of 1 molar ammonium acetate with a pH of 7.0. The total carbon (TC) and total nitrogen (TN) were analyzed using a VarioMax elemental analyzer (Elementar Analysensysteme GmbH, Langenselbold, Germany).
The total metal contents in the soil were determined spectrophotometrically according to the ISO procedure [ 19 ]. The plant material (aboveground parts) was dried at 60 °C, ground and ashed in a furnace at 450 °C for 6 h. The ash was dissolved in 5 mL of 6 mol∙dm 3 HCl and diluted to a constant volume with distilled water [ 20 ]. The obtained soil and plant extracts were analyzed to measure the metal contents by atomic absorption spectrophotometry (ASA) in a Varian Spectra AA220 FS apparatus. The method employed to determine the examined elements in all the extracts was atomic absorption spectrometry with atomisation in a flame of acetylene/air. To evaluate the validation of the obtained laboratory test results, the certified reference material RTH 907 was used. Recovery of the analyzed elements was achieved at the level of 94 to 98%.
To express the plants’ ability to take up and transport the most mobile and bioavailable amounts of heavy metals and to assess the actual site contamination with heavy metals the specified factor was calculated. The metal contents in the soil and vegetables were used to calculate the bioconcentration factors for individual metals. On the basis of heavy metal total contents in the soil and in cultivated plants the bioconcentration factors were calculated as follows [ 11 ]:
Additionally, the daily intake of metal (DIM) and the health risk index (HRI) were calculated. For the daily intake of metal (DIM) the following equation was used [ 16 ]:
*—based on WHO data [ 21 ] adults in Europe have an average daily consumption of carrots amounting to 2.8, tomatoes 44.1, cabbage 5.0 and beetroot 0.5 g/person
**—based on world statistics the average body weight of adults is 62.6 kg (average weight of men and women)
The health risk index (HRI) was calculated using the following equation given by Jan et al. [ 22 ], cited after [ 14 ]:
*—RfD—reference oral dose; the values for Cu, Zn, Pb, and Cd amounted to 0.04, 0.30, 0.004 and 0.001 (mg/kg/day), respectively (values given according to US-EPA [ 23 ]).
2.3. Statistical Analysis
The data presented in the paper are means of five replications. The data were compiled applying one-way ANOVA. Each of the parameters for individual vegetables was tested independently using the F-test at the significance level α = 0.95. The assumed null hypothesis was that the mean values of the examined parameter are equal for each of the analyzed vegetables against the alternative hypothesis that not all the means are equal. As a result of the rejection of the null hypothesis the least significant differences were calculated using the Tukey test at the significance level α = 0.05. Tukey’s analysis was performed to distinguish homogeneous groups among the analyzed parameters individually for vegetables. The data were analyzed using the STATOBL software working in the Windows environment. The obtained results are visualized in the form of boxplots. In a standardized way of displaying the distribution of data the minimal value, first quartile (Q1), median, third quartile (Q3), and maximal value are given.
The amounts of the analyzed heavy metals in the soils from individual studied zones decreased in the following sequence: III > IV > I > II. The quantitative differences between the minimum and maximum amounts were 5.0 times (Cu), 8 times (Cd), 12 times (Zn) and 16 times (Pb), which was statistically confirmed ( Table 2 ). Similar relationships can be noted with regard to the contents of heavy metals in the tested vegetables ( Table 2 ). The highest quantities of heavy metals were present in plants grown in the industrial zone and the lowest in plants grown in the suburbs. Analysed soils showed the highest amounts of Zn and the lowest of Cd and this directly influences the same relations in individual vegetables. It should be emphasized that the amounts of Pb and Cd in tomatoes grown in different zones did not differ significantly. It is worth noting that generally the amounts of metals found in vegetables cultivated in zones I and II did not differ statistically. A similar situation can be indicated in the case of plants grown in zones III and IV. Regardless of the zone, beetroots accumulated the highest, and tomatoes accumulated the lowest amounts of metals. As it was marked in the case of soils, also in relation to the tested vegetables the differences between the extreme values were determined, although they were not as spectacular as for soils. Regardless of the vegetable, the differences in the amount of Cu were 2–4 times, for Zn 1.5–2.0 times, for Pb 3.0 times, and for Cd 2.5–4.0 times ( Table 2 ).
Metal content in soils and plants in relation to individual areas of the city (mg·kg −1 ).
* I—central urban zone, II—suburban zone, III—industrial zone, IV—north-eastern industrial zone. ** The same letters mean no significant differences between the values.
On the basis of the metal amounts in the soil and the plants, the BCF value was calculated. BCF characterizes the theoretical transfer and concentration of a metal from the soil to the plant. Regardless of the cultivated plant and the analyzed metal, the lowest BCF values were determined in plants grown in the industrial zone (III) and the highest in the central urban zone (I). Independently of the analysed vegetables, the BCF values increased in the following sequence: Cu > Zn > Cd > Pb. Simultaneously one can notice that BCF values for plants, regardless of the metals and their amounts, decrease as follows: tomato < cabbage < carrot < beetroot. As can be seen from Figure 2 , for carrots the BCF average values for Cu ranged from 0.07 to 0.15, while for Zn it was from 0.01 to 0.09. Similar levels of values were recorded for BCF Cd (0.02–0.08). The mean values of BCF for Cu, Cd, and Zn found for carrots cultivated in the industrial zone significantly differed from those recorded in the other zones. No influence of heavy metals emitted by the local industry was found in relation to BCF for Pb (0.01–0.02), because the differences between the obtained values were non-significant.
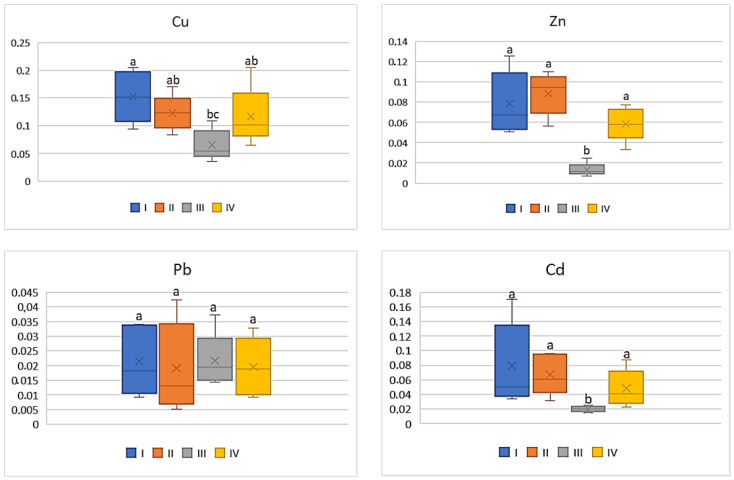
BCF values for carrots depending on the metal and study zones. The same letters mean no significant differences between the values.
Also, the industry had a weak influence on the differentiation of BCF mean values for the tested metals in tomato, because the differences were non-significant in the case of Cu (0.05–0.09) and Pb (0.007–0.01) ( Figure 3 ). The values of BCF for Zn and Cd calculated for tomatoes cultivated in zones I and II as well as III and IV did not differ statistically. The BCF values for Zn ranged from 0.01 to 0.06 and for Cd from 0.01 to 0.05.
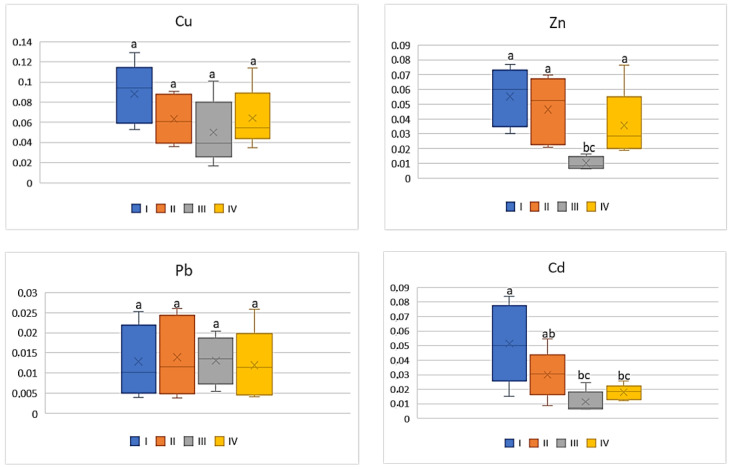
BCF values for tomato depending on the metal and study zones. The same letters mean no significant differences between the values.
For cabbage, similarly as for carrots or tomatoes, also low BCF values (0.008–0.1) were determined and it was independent of the metal. Simultaneously the data calculated for BCF Pb as well as for Cd were comparable between the individual zones where the discussed vegetable was grown ( Figure 4 ). The average values of BCF of Cu for cabbage from zone I (on average 0.1) were the highest and statistically different from the other calculated values (0.05–0.07). BCF values for Zn in cabbages grown in zone III (on average 0.01) were significantly lower compared to the values found for the discussed vegetable from the other zones (0.04–0.05) ( Figure 4 ).
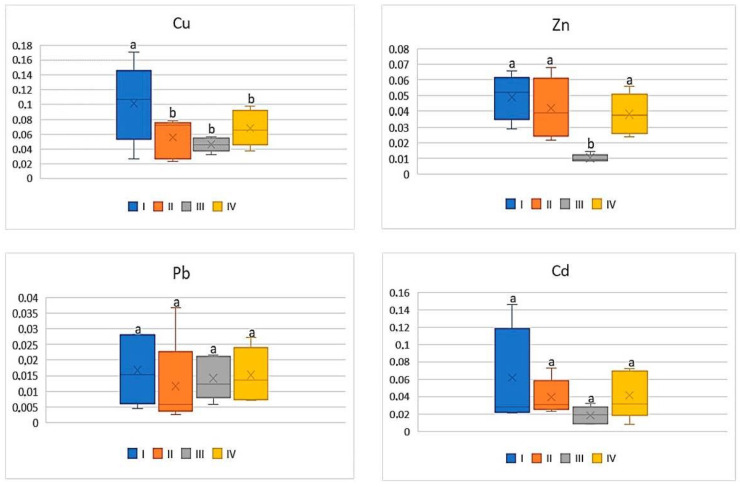
BCF values for cabbage depending on the metal and study zones. The same letters mean no significant differences between the values.
Of all the cultivated vegetables, the highest BCF mean values were found for beetroot ( Figure 5 ). The direct influence of industry in zone III was marked only in relation to Cu and Zn, for which the BCF values were statistically lower (on average 0.09 for Cu and 0.02 for Zn) compared to the other values (0.16–0.23 for Cu and 0.09–0.11 for Zn). The BCF values for Pb and Cd were similar (0.01–0.03 for Pb and 0.03–0.06) and did not differ statistically ( Figure 5 ).
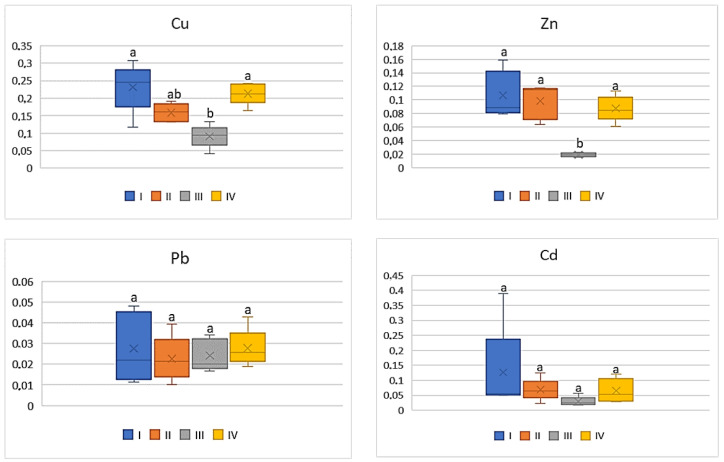
BCF values for beetroot depending on the metal and study zones. The same letters mean no significant differences between the values.
The intensity of the industry’s impact on the quality of vegetables should not only be assessed on the basis of metal concentrations in their edible parts, but the potential impact of heavy metals included in vegetables on human health should be also considered. Therefore, the influence of the anthropogenic factor on changes in DIM and HRI values was analyzed. Regardless of the tested vegetable and metal, the highest values of DIM and HRI were determined in plants grown in the industrial zone (III) and the lowest in the suburban zone (II). Moreover, the DIM values depended on the metal and increased in the following sequence: Cd < Pb < Zn < Cu. Also, in the case of HRI values, these values decrease as follows: Cu > Pb > Cd > Zn. Regardless of the analyzed metals, DIM values increase in vegetables in the following sequence: beetroot < cabbage < carrot < tomato. In the case of HRI values, one may notice a slightly different order: beetroot < cabbage < tomato < carrot. For carrot the DIM values for Cu ranged on average from 0.41 μg∙kg −1 to 1.02 μg∙kg −1 , for Zn it was from 0.87 μg∙kg −1 to 1.43 μg∙kg −1 , while the values of the discussed parameter in both cases did not differ statistically for plants from zones I and II and for zones III and IV. In turn, DIM for Pb and Cd in carrot showed lower mean values ranging from 0.04 μg∙kg −1 to 0.12 μg∙kg −1 (Pb) and from 0.005 μg∙kg −1 to 0.01 μg∙kg −1 (Cd) ( Figure 6 ).
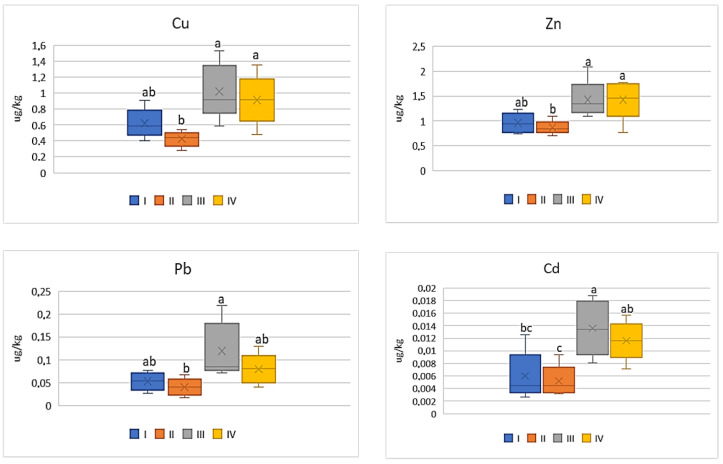
DIM values for carrot depending on the metal and study zones. The same letters mean no significant differences between the values.
As shown in Figure 7 , the HRI values determined for carrots indicated a significant differentiation depending on the analyzed metal. Regardless of the above, the significantly lower HRI values were found for carrots cultivated in zone II, as they were on average 10.62, 10.0, 5.19 and 2.88 for Cu, Pb, Cd, nd Zn, respectively. Carrots grown in the industrial zone had the highest HRI values. At the same time, it should be noted that the HRI values determined for zones III and IV did not differ significantly, similarly to those for zones I and II ( Figure 7 ).
HRI values for carrot depending on the metal and study zones. The same letters mean no significant differences between the values.
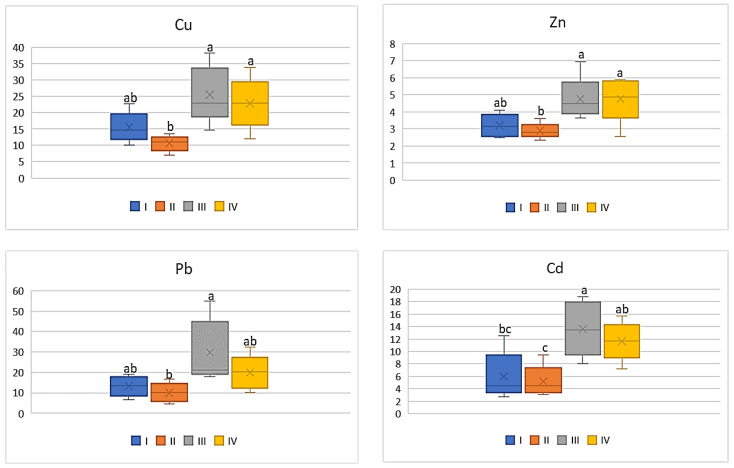
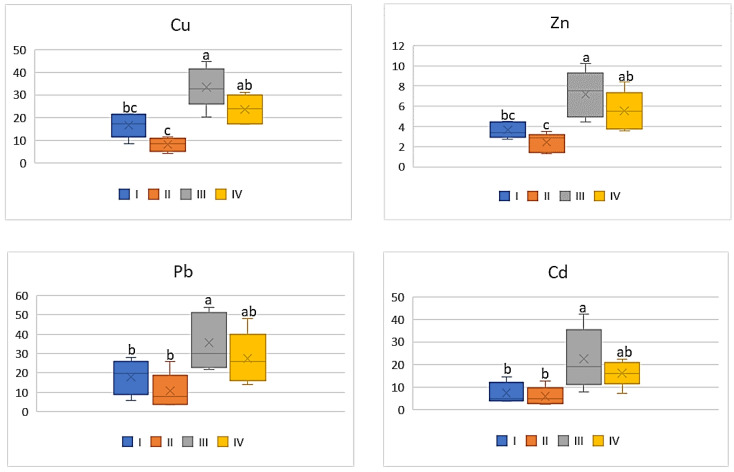
As shown in Figure 8 , the influence of industry emitted heavy metals on DIM values in tomato was strongly marked. The mean values of DIM for Cu and Zn determined for this vegetable grown in the industrial zone (III) differed significantly from the other data. Regardless of the above, the lowest values were determined for tomatoes representing zone II (3.76 μg∙kg −1 for Cu; 7.21 μg∙kg −1 for Zn; 0.5 μg∙kg −1 for Pb, and 0.04 μg∙kg −1 for Cd), and the highest for those from zone III (11.88 μg∙kg −1 for Cu; 18.08 μg∙kg −1 for Zn; 1.2 μg∙kg −1 for Pb 0.12 μg∙kg −1 for Cd) ( Figure 8 ). It is worth noticing that the DIM values calculated for Pb as well as Cd did not differ significantly.
DIM values for tomato depending on the metal and study zone. The same letters mean no significant differences between the values.
A similar relationship can be found in relation to HRI mean values in tomato ( Figure 9 ). The calculated HRI values for this plant grown in zone III significantly differed from those in the other zones of the study area, being the highest (Cu: 296.94; Zn: 60.26; Pb; 306.46; Cd: 122.58). The lowest values of HRI were found in tomatoes representing the suburban zone (Cu: 94.05; Zn: 24.05; Pb: 119.87; Cd: 38.04) ( Figure 9 ). Generally, the HRI values obtained for plants representing zones I, II, and IV did not differ significantly.
HRI values for tomato depending on the metal and study zones. The same letters mean no significant differences between the values.
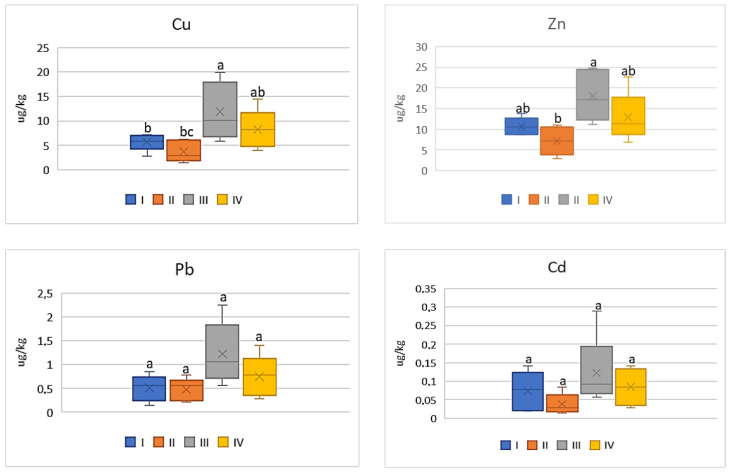
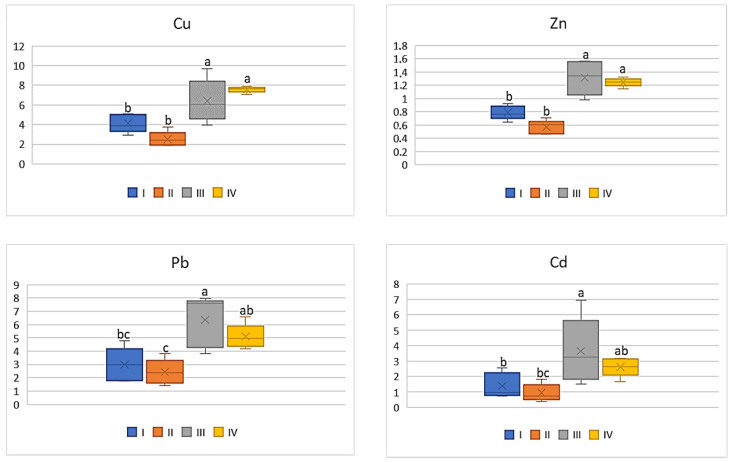
As shown by the data in Figure 10 , the DIM values of the analyzed metals for cabbage were significantly higher (4.0 times) for vegetables representing the industrial zone (III) compared to values calculated for plants cultivated in the other zones of the study area. The lowest values were recorded for cabbage from zone II: Cu—0.32 μg∙kg −1 ; Zn—0.73 μg∙kg −1 ; Pb—0.04 μg∙kg −1 ; and Cd—0.006 μg∙kg −1 . Similar relationships can be found in the case of HRI values, which for Cu ranged from 8.11 to 33.51, for Zn from 2.43 to 7.19, for Pb from 10.8 to 35.5, and for Cd from 5.91 to 22.52 ( Figure 11 ). The highest values were obtained for plants cultivated in the industrial area (III zone) and the lowest for the suburban area (II zone). Generally, the data calculated for vegetables from zones I; II, and III did not differ from one another.
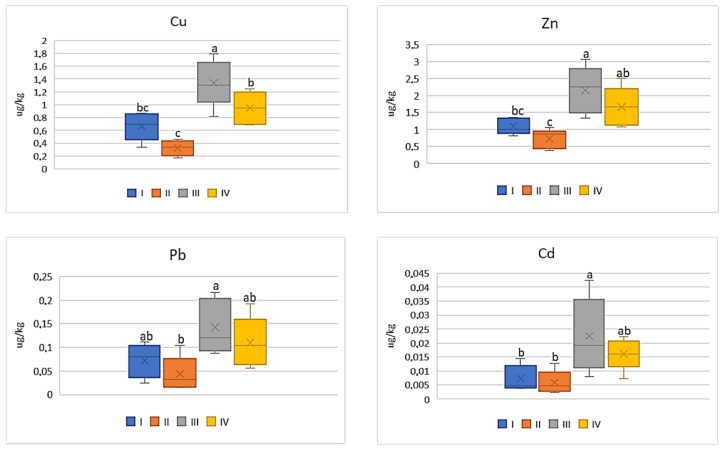
DIM values for cabbage depending on the metal and study zones. The same letters mean no significant differences between the values.
HRI values for cabbage depending on the metal and study zones. The same letters mean no significant differences between the values.
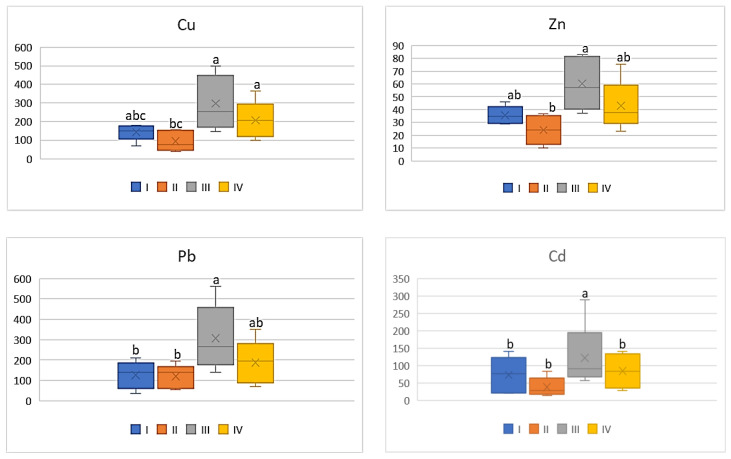
Among all the analyzed vegetables the lowest DIM and HRI values were determined for beetroot ( Figure 12 and Figure 13 ), which was a phenomenon characteristic of all the tested metals. Nevertheless, the impact of industry in zone III was noted, because the plants grown there had the highest DIM values (2–4.0 times) in relation to the values found for beetroots from zones I and II. The DIM mean values for Cu ranged from 0.1 μg∙kg −1 to 0.3 μg∙kg −1 , for Zn from 0.17 μg∙kg −1 to 0.39 μg∙kg −1 , for Pb from 0.009 μg∙kg −1 to 0.03 μg∙kg −1 , and for Cd from 0.001 μg∙kg −1 to 0.004 μg∙kg −1 ( Figure 12 ). Data calculated for Cu, Zn, and Pb for plants representing zones I and II as well as III and IV were comparable, so the differences were non-significant. A slightly different situation can be noticed in DIM values for Cd, because a significant difference was found only for plants from zones I and II ( Figure 12 ). According to the data in Figure 13 , the HRI average values for beetroots ranged from 2.52 to 7.57 (Cu), 0.57 to 1.31 (Zn), 2.4 to 6.4 (Pb), and 0.94 to 3.64 (Cd). Regardless of the analyzed metal, the maximum values for beetroots grown in zone III significantly differed from those minimum HRI values found for plants representing zone II. Additionally, it should be stated that the HRI values calculated for beetroots grown in zones I and II as well as III and IV were comparable ( Figure 13 ).
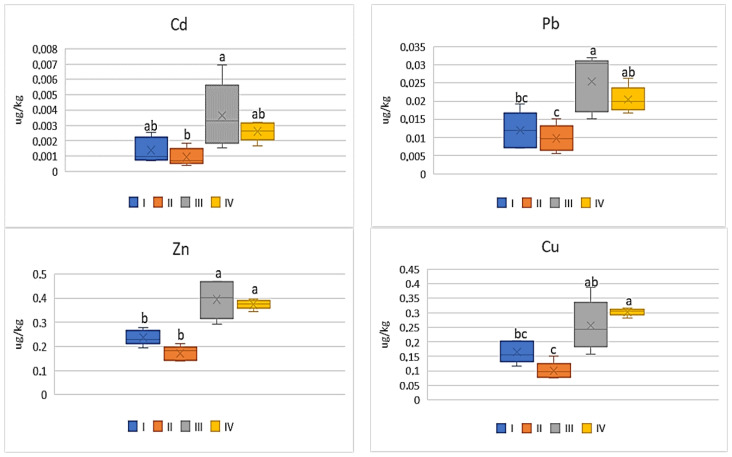
DIM values for beetroot depending on the metal and study zones. The same letters mean no significant differences between the values.
HRI values for beetroot depending on the metal and study zones. The same letters mean no significant differences between the values.
4. Discussion
The issue of horticulture in industrial cities can be controversial due to the potential contamination of crops with emissions from industrial plants. On the other hand, allotment gardens have a long tradition and are very popular in cities. Allotment gardens are a major form of urban agriculture in the world that act as an important feature of urban food security and sustainable urban food systems [ 24 , 25 ]. However, simultaneously such gardens are often exposed to several emission sources from the surrounding industry, which can lead to contamination of cultivated plants. Taking under consideration the possible pollution of soils and vegetables with heavy metals, this issue must be considered in terms of ecological risks and human health hazards. It is because soil acts as a reservoir of accumulated toxic metals, which are transferred to vegetables and enter the human food chain causing non-carcinogenic and carcinogenic health hazards due to consumption of contaminated vegetables [ 26 ]. Awareness of these dependencies is growing, although not at the same rate all over the world, as evidenced by the low interest in this subject in Kazakhstan. The diagnosis and analysis of this issue was carried out in a large industrial center—the city of Oskemen. According to Woszczyk et al. [ 27 ], the area of the city is exposed to the impact of polymetallic pollution and the contents of Cd, Cu, Pb, and Zn exceed values of the geochemical background for each element from moderate to extreme. According to Boluspaeva et al. [ 18 ], the average concentration of heavy metals in the soils of Oskemen exceeded the background level of studied metals in soils in this region (Cu—21.4 mg·kg −1 , Zn—67.4 mg·kg −1 , Pb—17.8 mg·kg −1 , Cd—0.2 mg·kg −1 ) from 2 to 13 times. This study confirms the information cited above, because the average content of the analyzed metals varies widely in soils. In comparison to the literature data [ 28 , 29 ] presented for other industrial areas the amounts of heavy metals found in soils of Oskemen were higher. Taking under consideration the fact that there are more than 30,000 summer cottages and private houses located in the city and these areas are mainly intended by the residents for growing vegetables, it makes sense to assess the metal contents in soils according to hygienic standards. To assess the degree of soil pollution in Kazakhstan, an indicator is used such as the multiplicity of exceeding the maximum permissible level of chemicals. According to the approved order of the Ministry of Health of the Republic of Kazakhstan [ 30 ], the multiplicity of exceeding the limit level <1 means that the soils are clean, the multiplicity of exceeding the permissible level from 1 to 10 corresponds to heavily polluted soils. The obtained average values of the analyzed metals in soils in all the studied zones exceeded the maximum permissible level (Cu—33 mg·kg −1 and for Pb—32 mg·kg −1 ) adopted in Kazakhstan [ 31 ] by 2 to 11 times, which corresponds to heavily polluted soils. The contents of Cu, Pb, Zn, and Cd in zones I and II of the city are below the levels recommended by the Directive [ 32 ], where the maximum allowable limit for the content of metals in soil is as follows: for Cu 300 mg·kg −1 , for Zn—300 mg·kg −1 , for Pb 140 mg·kg −1 , and for Cd 3 mg·kg −1 . The contents of all the analyzed metals found in the industrial zone (III) were 3- to 9-fold greater in comparison to the recommended permissible levels given by Directive [ 32 ]. It should be noted that the average contents of heavy metals in the soils decreased noticeably depending on the distance from the emission source. Thus, the amounts of metals in soils from the industrial area (zone III) were from 2 to 12 times higher than those in soils from zone II (suburban area). The relatively low metal contents in soils of the suburban zone can be related not only to the distance from the emission source (13,000 m from the source of emissions, see Table 1 ), but also to the opposite direction of the wind rose. A comparison of the results of this study with the literature data [ 33 , 34 ] shows that the contents of Cd, Pb, Zn, and Cu determined for the soils of industrial areas were more than 10 times higher. On the other hand, Chowdhury et al. [ 35 ] found Cd in the range of 3.22–18.79 mg·kg −1 in soils and these values are quite comparable to those obtained in this study in soils from the industrial area (6.33–15.2 mg·kg −1 ).
It should be noted that high heavy metal contents in the soils were correlated with those in plant products. The maximum contents of heavy metals were found in vegetables grown in zone III, and the minimum in zone II. These data are consistent with studies by Haque, et al. [ 36 ] or Zvolak [ 2 ], who noted significant heavy metal contamination in vegetables grown near industrial plants. Also, Zvolak [ 2 ], concluded that leaf and root crops are not suitable for growing in contaminated soils due to the high storage capacity of heavy metals. A significant risk of heavy metal-contaminated vegetables grown near industrial areas was confirmed by studies of Gupta et al. [ 4 ], Sun and Chen [ 37 ], Murray et al. [ 38 ], Sung and Park [ 39 ]. The range of heavy metal contents in vegetables grown on the soils of Oskemen vary greatly—from close to natural up to exceeding the hygienic standard proposed by FAO/WHO [ 40 ]. The safe limits for heavy metals in foodstuffs specified by the above-mentioned document are 73.0 mg·kg −1 for Cu, 99.0 mg·kg −1 for Zn, 0.3 mg·kg −1 for Pb, and 0.2 mg·kg −1 for Cd. When comparing these limits with the data presented in this study, it should be emphasized that the Cu and Zn amounts in the tested vegetables did not exceed the permissible level. At the same time, the contents of Cu and Zn in tomatoes were higher in comparison to results obtained by Haque et al. [ 36 ] or Chowdhury et al. [ 35 ]. Haque et al. [ 36 ] compared the Cu, Zn, Pb, and Cd contents in vegetables grown in industrial and non-industrial areas and found that significant quantitative differences clearly indicate the negative accumulation of metals in plants grown in industrial areas. These observations are confirmed in the presented paper. The outcomes from this study showed that Pb and Cd contents exceeded permissible thresholds [ 40 ] in vegetables grown in zones III and IV (with exception for Cd in tomato). The highest contents of Pb and Cd were recorded in beetroots and regardless of the cultivation site, the values were 2–11 times higher than the permissible levels for Cd and Pb, respectively. High Pb and Cd amounts in vegetables grown in an industrial area were also reported by Haque et al. [ 36 ] and by Gao et al. [ 28 ]. It should be underlined here that tomatoes accumulated the lowest Pb and Cd amounts, which in the case of Cd was also highlighted by Yang et al. [ 41 ]. The content of Cd in the analyzed vegetables was comparable to the results obtained by Gupta et al. [ 42 ], who determined from 0.14 to 0.23 mg·kg −1 of Cd for different vegetables. Moyo et al. [ 43 ] and Chowdhury et al. [ 35 ] found higher levels of Cd in tomatoes (0.6 mg·kg −1 and 0.62–2.88 mg·kg −1 , respectively, for the cited authors) and in cabbage (0.54 mg·kg −1 and 0.91–3.52 mg·kg −1 , respectively, for the cited authors) than in this study. It should be emphasized here that Cd and Pb are not essential elements for plant growth and can cause toxic effects in living organisms even at trace amounts [ 5 , 44 ].
The metal content in soils and plants provide general information concerning the current state of the environment, informing on their potential accumulation. However, when considering the negative impact of heavy metals on plants, especially on their edible parts, one should take into account their morphology and physiology, which will directly determine the individual intensity of heavy metal accumulation by a given plant [ 45 ]. Therefore, a more informative indicator allowing to reliably assess such a negative influence of metals and their accumulation intensity by plants seems to be provided by the bioconcentration factor (BCF). The bioconcentration factor (BCF) is widely used to assess toxicity of heavy metals as well as their translocation from soil to plants [ 11 , 46 , 47 , 48 ]. Additionally, Hu et al. [ 49 ] stated that the bioaccumulation values rather than the total contents of heavy metals should be taken into consideration, since more plants differ greatly in the degree of accumulation of heavy metals. According to Bounar et al. [ 50 ], a value of BCF < 0.1 indicates that vegetables are more successful at limiting the intake of heavy metals from contaminated soil. If values of BCF > 1, it shows that these plant species can be included in the category of heavy metal hyperaccumulators [ 51 ]. In this study, the range of BCF values, regardless of heavy metals and vegetables, amounted from 0.008 to 0.23, so they did not exceed 1. Despite differences between the minimum and maximum values of BCF, they depended on the vegetable and the zone in which the plant was grown. Regardless of the vegetable, the lowest values were recorded for zone III and the highest for zone II. Despite such differences, in most cases the values were statistically non-significant. The indicated differences in the BCF values for vegetables grown on soils from the II and III zones are the result of the amount of metals in the soil and in the plant. Generally, the higher the metal content found in the soil, the lower the BCF values were calculated for individual metals and vegetables. However, when interpreting this indicator, soil properties responsible for their sorption capacity, i.e., the potential binding of metals by soil colloids, should also be taken into account. Soils from zone II, compared to those from zone III, were characterized by lower CEC values and TC content (see Table 1 ). As indicated by Jakubus and Graczyk [ 10 ], the availability of metals for plants depends, among others, on such factors. Thus, it can be assumed that although in the soils from zone II, located further away from the emission source, the content of metals was lower, their bioavailability was higher, resulting in higher uptake of metals with crop yield and finally BCF value.
The lowest BCF values were determined for Pb (plant range: 0.008–0.01) and the highest for Cu (plant range: 0.05–0.23). Beetroot was characterized by the highest values of BCF (Cu: 0.09–0.23; Zn: 0.01–0.11; Cd: 0.03–0.013; Pb: 0.01–0.03), while cabbage had the smallest (Cu: 0.05–0.1; Zn: 0.01–0.05; Cd: 0.02–0.05; Pb: 0.008–0.02). The statement of Yang et al. [ 41 ] or Zhong et al. [ 52 ] that root plants have a high potential to accumulate heavy metals was proved by the outcomes of this study, because apart from beetroots high BCF values were also determined for carrots. This may be linked to the fact that the edible parts of these vegetables are in direct contact with contaminated soil. Although beetroot and carrot are classified as root plants, each plant belongs to a different botanical family (beetroot represents Amaranthaceae family and carrots Umbelliferae ), which has its consequences in the different physiology and biochemistry of these plants. This will directly affect the different nutritional needs of plants, different resistance to the presence of heavy metals in the environment and the production of biomass yield. In general, greater biomass is associated with greater intake of nutrients, both essential and harmful. This was showed in the present study, because beetroot was characterized by a greater accumulation and concentration of heavy metals compared to carrots, which was also reflected in higher BCF values for this vegetable. At the same time, the theory by Gupta et al. [ 4 ] and Hu et al. [ 49 ] that leafy vegetables accumulate greater amounts of metals in comparison to non-leafy vegetables was not confirmed, because the lowest BCF values were obtained for cabbage being a leafy vegetable. Analysis of data from the literature indicates that generally the BCF values were considerably higher than in present study. Latif et al. [ 14 ] showed higher Zn BCF values from 6.13 to 12.9, Cd BCF in the range of 1–7.8 and Cu BCF from 4.33 to 14 for different vegetables. Also Moyo et al. [ 43 ] reported higher values of BCF Cd for cabbage. The results obtained by Ashraf et. al. [ 33 ] showed greater BCF Pb values from 0.10 to 0.08 for different vegetables. In turn, Chowdhury et al. [ 35 ] reported BCF Cd values for tomatoes grown in industrial regions in the range from 0.07 to 0.27, while for cabbage they amounted from 0.12 to 0.35. Heavy metals may enter the human body in different ways, but consumption of contaminated food is the main route [ 14 , 53 ]. The DIM value indicates the transfer of heavy metals from plants to humans. In this study, the daily intake of metals with vegetables was calculated for adults and the results show that generally consumption of the analyzed vegetables containing heavy metals poses practically no risk in the case of single cases, as they are less than 1. An exception was found for tomatoes cultivated in soils from all the analyzed zones. The values of DIM for Cu varied from 3.76 to 11.88 μg·kg −1 and for Zn from 7.27 to 18.08 μg·kg −1 . Generally DIM values were significantly higher in zones III and IV rather than zones I and II. Haque et al. [ 36 ] compared DIM values for vegetables cultivated in industrial and non-industrial area and found considerably higher values in comparison to those calculated in this study. Regardless of the city zones, the calculated DIM values were the highest for Zn (range for plants: 0.17–18.08 μg·kg −1 ) and the lowest for Cd (range for plants: 0.001–0.12 μg·kg −1 ). Simultaneously for tomatoes the DIM values were the highest and for beetroots the lowest. Generally, the authors present significantly higher values of DIM calculated for vegetables. According to Latif et al. [ 14 ], the DIM of heavy metals for vegetables was 0.00166 (mg/day) for Cu, 0.0139 (mg/day) for Zn and 0.00012 (mg/day) for Cd. Higher DIM values for vegetables were shown in studies of Ashraf et al. [ 33 ], where DIM for tomatoes and cabbage for all metals amounted from 0.0002 to 0.0089 mg/day/kg weight. Data obtained by Mahmood and Malik [ 54 ] showed the DIM of heavy metals in the range from 0.0009 to 0.04 mg/day. Simultaneously the above-cited authors showed higher DIM values for beetroot or cabbage than for tomato and carrot. According to Latif et al. [ 14 ], PTDI amounted to 60 µg, 3 mg, 214 µg, and 60 mg per day for Cd, Cu, Pb, and Zn, respectively. Taking into account the above information and confronting it with the DIM values obtained in this study for metals, it can be stated that they were considerably lower than those given for PTDI. Therefore, it may be stated that the amounts of Cu, Zn, Cd, and Pb that could be theoretically consumed by adults with carrot, tomato, cabbage and beetroot products do not pose any health risk.
There are many paths by which people get exposed to potential toxic metal(oid)s, and ingestion of vegetables contaminated with such elements could damage human health [ 55 ]. Considering the complexity of the negative impact of heavy metals on human health, it is reasonable to thoroughly assess the potential threat to humans from heavy metals consumed with food, especially as the digestive route is the most popular way for harmful elements to enter the human body. According to Jakubus and Bakinowska [ 11 ] HRI is an important tool to assess the negative impact of heavy metals on human health. According to US-EPA [ 56 ], HRI > 1.0 is considered hazardous to human health. In this study, HRI values were considerably higher for zones III and IV than I and II. The same pattern was noted regarding the content of heavy metals in soils and vegetables. Simultaneously, the values of HRI for Cu, Zn, Cd, and Pb in all the zones exceeded 1.0, which can indicate a potential significant unfavourable influence on human health. An exception was only observed in the case of beetroots cultivated in the urban zones (I, II), where HRI Zn ranged from 0.57 to 0.79 and for HRI Pb varied from 0.3 to 0.64 independently of the city zone. Regardless of plants, the highest values were calculated for Cu (2.52–296.94) and the lowest for Pb (0.3–30.65). Moreover, for beetroots, small values were calculated (range for plants: 0.3–7.57), while for tomatoes they were the biggest (11.98–296.94), indicating that the level of non-carcinogenic adverse health effect is alarmingly higher than the safe limit. Calculations show that the HRI for Cd in studied vegetables was also found to be significantly higher than standard limits. Thus, the HRI of Cd in tomatoes ranges from 38 to 123, from 5 to 14 in carrots, from 6 to 23 in cabbage, and from 1 to 4 in beetroots. Cd is a non-essential metal and highly toxic. Cd has shown chronic effects including lung cancer, respiratory diseases, and hair and bone problems [ 42 ]. Therefore, it is urgent to take appropriate measures to reduce the concentration of heavy metals in the environment in the study region. Consumption of unsafe concentrations of heavy metals continuously through food may lead to chronic accumulation of heavy metals in the human kidney and liver, consequently disrupting numerous biochemical processes and leading to cardiovascular, nerve, kidney and bone diseases [ 45 ]. Hu et al. [ 57 ], noted that excessive bioaccumulation of toxic heavy metals in vegetables may result in the unavailability of dietary nutrients to humans or cause health problems for both humans and the ecosystem. According to Li et al. [ 58 ], exposure to lead (Pb) may cause plumbism, anaemia, nephropathy, gastrointestinal colic, and central nervous system symptoms. High HRI values for tomato were also reported by Gao et al. [ 28 ]. The HRI values obtained in this study are significantly higher than those given in the literature by Mahmood and Malik [ 54 ], where HRI values for the metals were at the level of 0.02–0.45, and Latif et al. [ 14 ], who obtained HRI values < 1. Galal et al. [ 59 ] noted that the HRI values for Pb and Cd calculated for plants from contaminated sites were many times greater than 1.0 and consumption of such a plant product will pose a danger for the health of the local population. Growing vegetables on contaminated soils directly affects the quality of the products and this problem is closely related to food security. Despite the fact that urban gardening has not only aesthetic, but also economic attractiveness for the population of the city of Oskemen, the results of this study show significant negative aspects. In the light of obtained results the authors of this study do not recommend the use of vegetables grown in industrial areas due to the increased contents of heavy metals, as long-term consumption of contaminated vegetables can have adverse effects on human health. The results obtained indicate the need for further research, including the impact of contaminated vegetables on children as well as ongoing monitoring of the metal contents in the edible parts of cultivated plants in industrialized regions.

5. Conclusions
The excessive anthropogenic load in the city of Oskemen led to a deterioration in the environmental situation in general. In this study, in the soils of industrial zones the contents of the analyzed metals were found to be above the permissible limits. Generally, with a small exception for Cu and Zn in tomato, the analyzed metals exceeded thresholds given by FAO/WHO. Despite this fact, the obtained BCF values for the tested vegetables were very low, indicating low efficiency of heavy metal transfer from soil to plants, limiting their uptake. The DIM values of heavy metals for cabbage, beetroots and carrots in this study were estimated to be below 1, which indicates that the analyzed vegetables grown in the city area present little or no risk when consumed once. On the other hand, the DIM for Cu and Zn in tomatoes exceeded safe limits. It was found that consumption of vegetables grown in the zones of Oskemen could pose a significant non-carcinogenic risk to consumers, which was confirmed by the recorded HRI values. In the light of the presented results, it is reasonable to assess the usefulness of the parameters used in this study. In the authors’ opinion the best forecasting and estimation of negative effects of heavy metals on human health are provided by the use of HRI. Additionally, the outcomes of this study will help in the future planning of vegetable cultivation and the formulation of policies to strengthen human health by reducing and mitigating of the negative effect of heavy metals released into the environment as a result of anthropogenic activity.
Author Contributions
Conceptualization, M.J.; methodology, L.B., A.A., Y.B., M.J. and W.S.; software, A.A., Y.B., L.B. and M.J.; validation, M.J., W.S. and L.B.; formal analysis, M.J.; investigation, L.B.,A.A. and Y.B.; resources, L.B., A.A. and Y.B.; data curation, M.J.; writing—original draft preparation, M.J. and L.B.; writing—review and editing, M.J. and L.B., visualization, M.J. and W.S.; supervision, M.J., W.S. and A.A.; project administration, A.A. and L.B.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
- 1. Selvi A., Rajasekar A., Theerthagiri J., Ananthaselvam A., Sathishkumar K., Madhavan J., Rahman P.K.S.M. Integrated Remediation Processes Toward Heavy Metal Removal/Recovery from Various Environments—A review. Front. Environ. Sci. 2019;7:66. doi: 10.3389/fenvs.2019.00066. [ DOI ] [ Google Scholar ]
- 2. Zwolak A., Sarzyńska M., Szpyrka E., Stawarczyk K. Sources of Soil Pollution by Heavy Metals and Their Accumulation in Vegetables: A Review. Water Air Soil Poll. 2019;230:164. doi: 10.1007/s11270-019-4221-y. [ DOI ] [ Google Scholar ]
- 3. Hou D., O’Connor D., Igalavithana A.D., Alessi D.S., Luo J., Tsang D.C.W., Sparks D.L., Yamauchi Y., Rinklebe J., Ok Y.S. Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 2020;1:366–381. doi: 10.1038/s43017-020-0061-y. [ DOI ] [ Google Scholar ]
- 4. Gupta N., Yadav K.K., Kumar V., Kumar S., Chadd R.P., Kumar A. Trace elements in soil-vegetables interface: Translocation, bioaccumulation, toxicity and amelioration-A review. Sci. Total Environ. 2019;651:2927–2942. doi: 10.1016/j.scitotenv.2018.10.047. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Sandeep G., Vijayalatha K.R., Anitha T. Heavy metals and its impact in vegetable crops. Int. J. Chem. Stud. 2019;7:1612–1621. [ Google Scholar ]
- 6. Nag R., O’Rourke S.M., Cummins E. Risk factors and assessment strategies for the evaluation of human or environmental risk from metal(loid)s—A focus on Ireland. Sci. Total Environ. 2022;802:149839. doi: 10.1016/j.scitotenv.2021.149839. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Vanisree C.R., Sankhla M.S., Singh P., Jadhav E.B., Verma R.K., Awasthi K.K., Awasthi G., Naga V. Heavy metal contamination of food crops: Transportation via food chain, human consumption, toxicity and management strategies. In: Salek H., Hassam A., editors. Environmental Impact and Remediation of Heavy Metals. IntechOpen; London, UK: 2022. [ Google Scholar ]
- 8. Manzoor J., Sharma M., Wani K.A. Heavy metal in vegetables and their impact on the nutrient quality of vegetables: A review. J. Plant Nutr. 2018;41:1744–1763. doi: 10.1080/01904167.2018.1462382. [ DOI ] [ Google Scholar ]
- 9. Ćwieląg-Drabek M., Piekut A., Gut K., Grabowski M. Risk of cadmium, lead and zinc exposure from consumption of vegetables produced in areas with mining and smelting past. Sci. Rep. 2020;10:3363. doi: 10.1038/s41598-020-60386-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Jakubus M., Graczyk M. The Effect of Compost and Fly Ash Treatment of Contaminated Soil on the Immobilisation and Bioavailability of Lead. J. Agronomy. 2021;11:1188. doi: 10.3390/agronomy11061188. [ DOI ] [ Google Scholar ]
- 11. Jakubus M., Bakinowska E. The effect of immobilizing agents on Zn and Cu availability for plants in relation to their potential health risk. Appl. Sci. 2022;12:6538. doi: 10.3390/app12136538. [ DOI ] [ Google Scholar ]
- 12. Kumar V., Pandita S., Sharma A., Bakshi P., Sharna P., Karaouzas I., Bhardwaj R., Thukral A.K., Cerda A. Ecological and human health risks appraisal of metal(loid)s in agricultural soils: A review. Geol. Ecol. Landsc. 2021;5:173–185. doi: 10.1080/24749508.2019.1701310. [ DOI ] [ Google Scholar ]
- 13. Gupta N., Yadaw K.K., Kumar V., Krishnan S., Kumar S., Nejad Z.D., Khan M.A., Alam J. Evaluating heavy metals contamination in soil and vegetables in the region of North India: Levels, transfer and potential human health risk analysis. Environ. Toxicol. Pharmacol. 2021;82:103563. doi: 10.1016/j.etap.2020.103563. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Latif A., Bilal M., Asghar W., Azeem M., Ahmad M.I., Abbas A., Ahmad M.Z., Shahzad T. Heavy metal accumulation in vegetables and assessment of their potential health risk. J. Environ. Anal. Chem. 2018;5:234. doi: 10.4172/2380-2391.1000234. [ DOI ] [ Google Scholar ]
- 15. Guo G., Zhang D., Wang Y. Probabilistic human health risk assessment of heavy metal intake via vegetable consumption around Pb/Zn smelters in southwest China. Int. J. Environ. Res. Public Health. 2019;16:3267. doi: 10.3390/ijerph16183267. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Leblebeci Z., Kar M. Heavy metals accumulation in vegetables irrigated with different water sources and their human daily intake in Nevsehir. J. Agric. Sci. Technol. 2018;20:401–415. [ Google Scholar ]
- 17. IUSS Working Group WRB . World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. 4th ed. International Union of Soil Sciences (IUSS); Vienna, Austria: 2022. [ Google Scholar ]
- 18. Boluspaeva L., Panin M., Mocek A., Spychalski W. Heavy metals in soils of the town of Ust-Kamenogorsk in the Republic of Kazakhstan. Soil Sci. Annu. 2011;62:40–47. [ Google Scholar ]
- 19. Soil Quality-Extraction of Trace Elements Soluble in Aqua Regia. International Organization of Standardization; Genève, Switzerland: 1995. [ Google Scholar ]
- 20. Ostrowska A., Gawliński S., Szczubialka Z. Methods for Analysis and Evaluation of Soil and Plant Properties. 1st ed. IOŚ Warszawa; Warszawa, Poland: 1991. pp. 158–167. (In Polish) [ Google Scholar ]
- 21. WHO Food Safety Issue. GEMS/FOOD. Regional Diets. Food Safety Department WHO. 2003. [(accessed on 22 November 2022)]. Available online: https://apps.who.int/iris/handle/10665/42833 .
- 22. Jan F.A., Ishaq M., Khan S., Ihsanullah I., Ahmad I., Schakirullah H. A comparative study of human health risks via consumption of food crops grown on wastewater irrigated soil (Peshawar) and relatively clean water irrigated soil (lower Dir) J. Hazard. Mater. 2010;179:612–621. doi: 10.1016/j.jhazmat.2010.03.047. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. US-EPA Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures. EPA/630/R-00/002. [(accessed on 18 November 2022)];2000 Available online: https://ordspub.epa.gov/ords/eims/eimscomm.getfile?p_download_id=4486 .
- 24. Crispo M., Dosbon M.C., Blevins R.S., Meredith W., Lake J.A., Edmonson J.L. Heavy metals and metalloids concentrations across UK urban horticultural soils and the factors infuencing their bioavailability to food crops. Environ. Pollut. 2021;288:117960. doi: 10.1016/j.envpol.2021.117960. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Tobarra M.A., López L.A., Cadarso M.A., Gómez N., Cazcarro I. Is seasonal households’ consumption good for the nexus carbon/water footprint? Te Spanish fruits and vegetables case. Environ. Sci. Technol. 2018;52:12066–12077. doi: 10.1021/acs.est.8b00221. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Proshad R., Kormoker T., Islam S.M., Chandra K. Potential health risk of heavy metals via consumption of rice and vegetables grown in the industrial areas of Bangladesh. Hum. Ecol. Risk Assess. 2019;26:921–943. doi: 10.1080/10807039.2018.1546114. [ DOI ] [ Google Scholar ]
- 27. Woszczyk M., Spychalski W., Boluspaeva L. Trace metal (Cd, Cu, Pb, Zn) fractionation in urban-industrial soils of Ust-Kamenogorsk (Oskemen), Kazakhstan—Implications for the assessment of environmental quality. Environ. Monit. Assess. 2018;190:362. doi: 10.1007/s10661-018-6733-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Gao J., Zhang D., Proshad R., Uwiringiyimana E., Wang Z. Assessment of the pollution levels of potential toxic elements in urban vegetable gardens in southwest China. Sci. Rep. 2021;11:22824. doi: 10.1038/s41598-021-02069-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Laaouidi Y., Bahmed A., Naylo A., El Khalil H., Ouvrard S., Schwartz C., Boularbah A. Trace Elements in Soils and Vegetables from Market Gardens of Urban Areas in Marrakech City. Biol. Trace Elem. Res. 2020;195:301–316. doi: 10.1007/s12011-019-01849-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Order of the Minister of Health of the Republic of Kazakhstan dated 21 April 2021. On Approval of the Hygienic Standards for the Safety of the Environment. [(accessed on 19 December 2022)]. Available online: https://adilet.zan.kz/rus/docs/V2100022595 .
- 31. Standards for Maximum Permissible Concentrations of Harmful Substances, Harmful Organisms and Other Biological Substances that Pollute the Soil, Approved by the Joint Order of the Ministry of Health of the Republic of Kazakhstan Dated 30 January 2004 № 99 and the Ministry of Environmental Protection of the Republic of Kazakhstan dated January 27, 2004. [(accessed on 19 December 2022)]. Available online: https://online.zakon.kz/Document/?doc_id=1046570&pos=32;-47#pos=32;-47 .
- 32. Directive 86/278/EWG. [(accessed on 22 November 2022)]. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31986L0278 .
- 33. Ashraf I., Ahmad F., Sharif A., Altaf A.R., Teng H. Heavy Metals Assessment in Water, Soil, Vegetables and Their Associated Health Risks via Consumption of Vegetables, District Kasur, Pakistan. SN Appl. Sci. 2021;3:552. doi: 10.1007/s42452-021-04547-y. [ DOI ] [ Google Scholar ]
- 34. Moghtaderi T., Mahmoudi S., Shakeri A., Masihabadi H.M. Heavy Metals Contamination and Human Health Risk Assessment in Soils of an Industrial Area, Bandar Abbas—South Central Iran. Hum. Ecol. Risk Assess. 2018;24:1058–1073. doi: 10.1080/10807039.2017.1405723. [ DOI ] [ Google Scholar ]
- 35. Chowdhury M.A.H., Chowdhury T., Rahman M.A. Heavy metal accumulation in tomato and cabbage grown in some industrially contaminated soils of Bangladesh: Heavy metals in contaminated soils and vegetables. J. Bangladesh Agric. Univ. 2019;17:288–294. doi: 10.3329/jbau.v17i3.43198. [ DOI ] [ Google Scholar ]
- 36. Haque M.M., Niloy M.N., Khirul A.M., Alam F.M., Tareq M.S. Appraisal of Probabilistic Human Health Risks of Heavy Metals in Vegetables from Industrial, Non-industrial and Arsenic Contaminated Areas of Bangladesh. Heliyon. 2021;7:e06309. doi: 10.1016/j.heliyon.2021.e06309. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Sun Z., Chen J. Risk Assessment of Potentially Toxic Elements (PTEs) Pollution at a Rural Industrial Wasteland in an Abandoned Metallurgy Factory in North China. Int. J. Environ. Res. Public Health. 2018;15:85. doi: 10.3390/ijerph15010085. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Murray H., Pinchin T.A., Macfie S.M. Compost application affects metal uptake in plants grown in urban garden soils and potential human health risk. J. Soils Sediments. 2011;11:815–829. doi: 10.1007/s11368-011-0359-y. [ DOI ] [ Google Scholar ]
- 39. Sung Y.C., Park C.B. The effect of site- and landscape-scale factors on lead contamination of leafy vegetables grown in urban gardens. Lands. Urban Plan. 2018;177:38–46. doi: 10.1016/j.landurbplan.2018.04.013. [ DOI ] [ Google Scholar ]
- 40. Food additives and contaminants, Joint Codex Alimentarius Commission, FAO/WHO. Food standards Programme, FAO/WHO; Geneve, Switzerland: 2001. [ Google Scholar ]
- 41. Yang Y., Zhang F.S., Li H.F., Jiang R.F. Accumulation of cadmium in the edible parts of six vegetable species grown in Cd-contaminated soils. J. Environ. Manag. 2009;90:1117–1122. doi: 10.1016/j.jenvman.2008.05.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Gupta N., Yadav K.K., Kumar V., Prasad S., Cabral-Pinto M.M.S., Jeon B.-H., Kumar S., Abdellattif M.H., Alsukaibia A.K.D. Investigation of Heavy Metal Accumulation in Vegetables and Health Risk to Humans from Their Consumption. Front. Environ. Sci. 2022;10:791052. doi: 10.3389/fenvs.2022.791052. [ DOI ] [ Google Scholar ]
- 43. Moyo B., Matodzi V., Legodi A.M., Pakade V.E., Tavengwa N.T. Determination of Cd, Mn and Ni accumulated in fruits, vegetables and soil in the Thohoyandou town area, South Africa. Water S.A. 2020;46:285–290. doi: 10.17159/wsa/2020.v46.i2.8244. [ DOI ] [ Google Scholar ]
- 44. Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Zhou H., Yang W., Zhou X., Liu L., Gu J., Wang W., Zou J., Tian T., Peng P., Liao B. Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. Int. J. Environ. Res. Public Health. 2016;13:289. doi: 10.3390/ijerph13030289. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Jakubus M., Bakinowska E., Tatuśko-Krygier N. Compost utilisation in a heavy metal immobilisation process evaluated by bioconcentration factors. J. Elem. 2019;24:1291–1307. doi: 10.5601/jelem.2018.23.4.1761. [ DOI ] [ Google Scholar ]
- 47. Rai K.P., Lee S.S., Zhan M., Tsang Y.F., Kim K.-H. Heavy metals in food crops: Health, risk, fate, mechanism and management. Environ. Int. 2019;125:365–385. doi: 10.1016/j.envint.2019.01.067. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Lu Y., Li X., He M., Zeng F., Li X. Accumulation of heavy metals in native plants growing on mining-influenced sites in Jinchang: A typical industrial city (China) Environ. Earth Sci. 2017;76:446–461. doi: 10.1007/s12665-017-6779-2. [ DOI ] [ Google Scholar ]
- 49. Hu B., Jia X., Hu J., Xu D., Xia F., Li Y. Assessment of heavy metal pollution and health risks in the soil—plant—human system in the Yangtze River Delta, China. Int. J. Environ. Res. Public Health. 2017;14:1042. doi: 10.3390/ijerph14091042. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Bounar A., Boukaka K., Leghouchi E. Determination of Heavy Metals in Tomatoes Cultivated under green Houses and Human Health Risk Assessment. Qual. Assur. Saf. Crops Foods. 2020;12:76–86. doi: 10.15586/QAS2019.639. [ DOI ] [ Google Scholar ]
- 51. Al-Qahtani K. Assessment of Heavy Metals Accumulation in Native Plant Species from Soils Contaminated in Riyadh City, Saudi Arabia. Life Sci. 2012;9:384–392. [ Google Scholar ]
- 52. Zhong T., Xue D., Zhao L., Zhang X. Concentration of heavy metals in vegetables and potential health risk assessment in China. Environ. Geochem. Health. 2018;40:313–322. doi: 10.1007/s10653-017-9909-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Zeng X., Wang Z., Wang J., Guo J., Chen X., Zhuang J. Health risk assessment of heavy metals via dietary intake of wheat grown in Tianjin sewage irrigation area. Ecotoxicology. 2015;24:2115–2124. doi: 10.1007/s10646-015-1547-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Mahmood A., Malik N.R. Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore, Pakistan. Arab. J. Chem. 2014;7:91–99. doi: 10.1016/j.arabjc.2013.07.002. [ DOI ] [ Google Scholar ]
- 55. Alsafran M., Usman K., Rizwan M., Ahmed T., Al Jabri H. The Carcinogenic and Non-Carcinogenic Health Risks of Metal(oid)s Bioaccumulation in Leafy Vegetables: A Consumption Advisory. Front. Environ. Sci. 2021;9:742269. doi: 10.3389/fenvs.2021.742269. [ DOI ] [ Google Scholar ]
- 56. US-EPA . Reference dose (RfD): Description and use in Health Risk Assessments. Background Document 1A, Integrated Risk Information System (IRIS) United States Environmental Protection Agency; Washington, DC, USA: 2012. [ Google Scholar ]
- 57. Hu J., Wu F., Wu S., Cao Z., Lin X., Wong M. Bioaccessibility, dietary exposure and human risk assessment of heavy metals from market vegetables in Hong Kong revealed with an in vitro gastrointestinal model. Chemosphere. 2013;91:455–461. doi: 10.1016/j.chemosphere.2012.11.066. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Li Z.Y., Ma Z.W., Kuijp T.J., Yuan Z.W., Huang L.S. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014;468:843–853. doi: 10.1016/j.scitotenv.2013.08.090. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Galal T.M., Hassan L.M., Ahmed D.A., Alamri S.A.M., Alrumman S.A., Eid E.M. Heavy metals uptake by the global economic crop (Pisum sativum L.) grown in contaminated soils and its associated health risks. PLoS ONE. 2021;16:e0252229. doi: 10.1371/journal.pone.0252229. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
- View on publisher site
- PDF (5.8 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Advertisement
Heavy metals in vegetables: a review of status, human health concerns, and management options
- Environmental Toxicology: Impact on Human Health
- Published: 03 August 2022
- Volume 30 , pages 71940–71956, ( 2023 )
Cite this article

- Seema Manwani 1 ,
- Pooja Devi 1 ,
- Tanvi Singh 2 ,
- Chandra Shekhar Yadav 1 , 4 ,
- Kumud Kant Awasthi 1 ,
- Narain Bhoot 3 &
- Garima Awasthi ORCID: orcid.org/0000-0002-0038-1240 1
1697 Accesses
11 Citations
Explore all metrics
For sustainable global growth, food security is a prime concern issue, both quantitatively and qualitatively. Adverse effects on crop quality from contaminants like heavy metals have affected food security and human health. Vegetables comprise the essential and nutritious part of the human diet as they contain a lot of health-promoting minerals and vitamins. However, the inadvertent excess accumulation of heavy metals (As, Cd, Hg, and Pb) in vegetables and their subsequent intake by humans may affect their physiology and metabolomics and has been associated with diseases like cancer, mental retardation, and immunosuppression. Many known sources of hazardous metals are volcano eruptions, soil erosion, use of chemical fertilizers in agriculture, the use of pesticides and herbicides, and irrigation with wastewater, industrial effluents, etc . that contaminate the vegetables through the soil, air and water. In this review, the problem of heavy metal contamination in vegetables is discussed along with the prospective management strategies like soil amendments, application of bioadsorbents, membrane filtration, bioremediation, and nanoremediation.
Graphical abstract
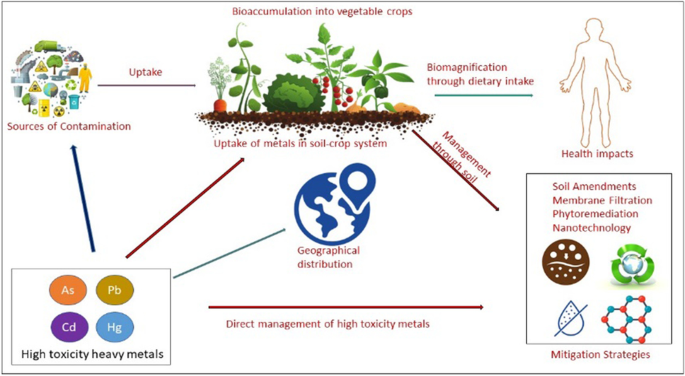
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Metal Toxicity to Certain Vegetables and Bioremediation of Metal-Polluted Soils

Production of Safer Vegetables from Heavy Metals Contaminated Soils: The Current Situation, Concerns Associated with Human Health and Novel Management Strategies
The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: a review, explore related subjects.
- Environmental Chemistry
Data availability
Not applicable.
Abbas K, Znad H, Awual MR (2018) A ligand anchored conjugate adsorbent for effective mercury II detection and removal from aqueous media. Chem Eng J 334:432–443. https://doi.org/10.1016/j.cej.2017.10.054
Article CAS Google Scholar
Abbasi AM, Iqbal J, Khan MA, Shah MH (2013) Health risk assessment and multivariate apportionment of trace metals in wild leafy vegetables from Lesser Himalayas, Pakistan. Ecotoxicol Environ Saf 9:237–244. https://doi.org/10.1016/j.ecoenv.2013.02.011
Abdul KSM, Jayasinghe SS, Chandana EP, Jayasumana C, De Silva PMC (2015) Arsenic and human health effects: A review. Environ Toxicol Pharmacol 403:828–846. https://doi.org/10.1016/j.etap.2015.09.016
Ahumada I, Sepúlveda K, Fernández P (2014) Effect of biosolid application to Mollisol Chilean soils on the bioavailability of heavy metals Cu, Cr, Ni, and Zn as assessed by bioassays with sunflower Helianthus annuus and DGT measurements. J Soils Sediments 14:886–896. https://doi.org/10.1007/s11368-013-0842-8
Ali H, Naseer M, Sajad MA (2012) Phytoremediation of heavy metals by Trifolium alexandrinum. Int J Environ Sci 2(3):1459–1469. https://doi.org/10.6088/ijes.002020300031
Alka S, Shahir S, Ibrahim N, Ndejiko MJ, Vo DVN, Abd Manan F (2020) Arsenic removal technologies and future trends: A mini review. J Clean Prod 278:123805. https://doi.org/10.1016/j.jclepro.2020.123805
Alloway BJ (2013) Sources of heavy metals and metalloids in soils. Heavy metals in soils. Springer, Dordrecht, pp 11–50. https://doi.org/10.1007/978-94-007-4470-7_2
Chapter Google Scholar
Anastopoulos I, Pashalidis I, Hosseini-Bandegharaei A, Giannakoudakis DA, Robalds A, Usman M, Escudero LB, Zhou Y, Colmenares JC, Núnez-Delgado A, Lima EC (2019) Agricultural biomass/waste as adsorbents for toxic metal decontamination of aqueous solutions. J Mol Liq 295:111684. https://doi.org/10.1016/j.molliq.2019.111684
Andreoli V, Sprovieri F (2017) Genetic aspects of susceptibility to mercury toxicity: an overview. Int J Environ Res Public Health 141:93. https://doi.org/10.3390/ijerph14010093
Antoniadis V, Levizou E, Shaheen SM, Ok YS, Sebastian A, Baum C, ... Rinklebe J (2017) Trace elements in the soil-plant interface: phytoavailability, translocation, and phytoremediation–a review. Earth-Sci Rev 171:621-645 https://doi.org/10.1016/j.earscirev.2017.06.005
Ara A, Usmani JA (2015) Lead toxicity: a review. Interdiscip Ttoxico 82:55–64. https://doi.org/10.1515/2Fintox-2015-0009
Article Google Scholar
Argos M, Ahsan H, Graziano JH (2012) Arsenic and human health: epidemiologic progress and public health implications. Rev Environ Health 274:191–195. https://doi.org/10.1515/reveh-2012-0021
Arreghini S, de Cabo L, Serafini R, de Iorio AF (2017) Effect of the combined addition of Zn and Pb on partitioning in sediments and their accumulation by the emergent macrophyte Schoenoplectus californicus. Environ Sci Pollut Res 249:8098–8107. https://doi.org/10.1007/s11356-017-8478-7
Ashrafi O, Yerushalmi L, Haghighat F (2015) Wastewater treatment in the pulp-and-paper industry: A review of treatment processes and the associated greenhouse gas emission. J Environ Manag 158:146–157. https://doi.org/10.1016/j.jenvman.2015.05.010
Awasthi G, Singh T, Awasthi A, Awasthi KK (2020a) Arsenic in mushrooms, fish, and animal products. Arsenic in Drinking Water and Food. Springer, Singapore, pp 307–323. https://doi.org/10.1007/978-981-13-8587-2_10
Awasthi G, Singh T, Tiwari Y, Awasthi A, Tripathi RD, Shrivastava S, Vajpayee P, Kumar A, Awasthi KK (2020b) A review on nanotechnological interventions for plant growth and production. Materials Today: Proceedings 31:685–93
Awasthi G, Nagar V, Mandzhieva S, Minkina T, Sankhla MS, Pandit PP, Aseri V, Awasthi KK, Rajput VD, Bauer T, Srivastava S (2022) Sustainable amelioration of heavy metals in soil ecosystem: Existing developments to emerging trends. Minerals 12(1):85
Bao S, Li K, Ning P, Peng J, Jin X, Tang L (2017) Highly effective removal of mercury and lead ions from wastewater by mercaptoamine-functionalised silicacoated magnetic nano-adsorbents: Behaviours and mechanisms. Appl Surf Sci 393:457–466. https://doi.org/10.1016/j.apsusc.2016.09.098
Bauddh, Singh RP (2012) Growth, tolerance efficiency and phytoremediation potential of Ricinus communis L. and Brassica juncea L. in salinity and drought affected cadmium contaminated soil. Ecotoxigy Environ Saf 85(13–22):H21. https://doi.org/10.1016/j.ecoenv.2012.08.019
Beckers F, Rinklebe J (2017) Cycling of mercury in the environment: sources, fate, and human health implications: A review. Crit Rev Environ Sci Technol 479:693–794. https://doi.org/10.1080/10643389.2017.1326277
Behera UK, France J (2016) Integrated farming systems and the livelihood security of small and marginal farmers in India and other developing countries. Adv Agron 138:235–282. https://doi.org/10.1016/bs.agron.2016.04.001
Bernhoft RA (2012) Mercury toxicity and treatment: a review of the literature. J Environ Public Health. https://doi.org/10.1155/2012/460508
Bradl HB (2004) Adsorption of heavy metal ions on soils and soils constituents. J Colloid Interface Sci 277(1):1–18. https://doi.org/10.1016/j.jcis.2004.04.005
Cañas JE, Long M, Nations S, Vadan R, Dai L, Luo M, ... Olszyk D 2008 Effects of functionalized and nonfunctionalized single‐walled carbon nanotubes on root elongation of select crop species. Environ Toxicol Chem: An Int J 27(9) https://doi.org/10.1897/08-117.1
Cao X, Alabresm A, Chen YP, Decho AW, Lead J (2020) Improved metal remediation using a combined bacterial and nanoscience approach. Sci Total Environ 704:135378. https://doi.org/10.1016/j.scitotenv.2019.135378
Chakraborti D, Rahman MM, Das B, Chatterjee A, Das D, Nayak B, Kumar M (2017) Groundwater arsenic contamination and its health effects in India. Hydrogeol J 254:1165–1181. https://doi.org/10.1007/s10040-017-1556
Chary NS, Kamala CT, Raj DSS (2008) Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol Environ Saf 693:513–524. https://doi.org/10.1016/j.ecoenv.2007.04.013
Chen L, Li F, Wei Y, Li G, Shen K, He HJ (2019) High cadmium adsorption on nanoscale zero-valent iron coated Eichhornia crassipes biochar. Environ Chem Lett 171:589–594. https://doi.org/10.1007/s10311-018-0811-y
Chen X, Wu C, Tang J, Hu S (2005) Arbuscular mycorrhizae enhance metal lead uptake and growth of host plants under a sand culture experiment. Chemosphere 60:665–671. https://doi.org/10.1016/j.chemosphere.2005.01.029
Chunhabundit R (2016) Cadmium exposure and potential health risk from foods in contaminated area, Thailand. Toxicol Res 321:65–72. https://doi.org/10.5487/TR.2016.32.1.065
Cobb GP, Sands K, Waters M, Wixson BG, Dorward-King E (2000) Accumulation of heavy metals by vegetables grown in mine wastes. Environ Toxicol Chem: An Int J 19(3):600–607. https://doi.org/10.1002/etc.5620190311
Corsi I, Winther-Nielsen M, Sethi R, Punta C, Della Torre C, Libralato G, Buttino I (2018) Ecofriendly nanotechnologies and nanomaterials for environmental applications: Key issue and consensus recommendations for sustainable and ecosafe nanoremediation. Ecotoxicol Environ Saf 154:237–244. https://doi.org/10.1016/j.ecoenv.2018.02.037
d’Aquino L, Staiano M, Gambale E, Basile A, Tommasi F (2018) Uptake and distribution of several inorganic ions in Nephrolepis cordifolia L. C. Presl grown on contaminated soil. Plant Biosyst-An International Journal Dealing with All Aspects of Plant Biology 1521:59–69. https://doi.org/10.1080/11263504.2016.1245217
Das B, Pandit MK, Ray K, Bhattacharyya K, Pari A, Sidhya P (2016) Impact of irrigation and organic matter amendments on arsenic accumulation in selected vegetables. Plant, Soil Environ 62(6):266–273. https://doi.org/10.17221/363/2015-PSE
Dhama K, Sachan S, Khandia R, Munjal A, Iqbal MNH, Latheef SK, Dadar M (2016) Medicinal and beneficial health applications of Tinospora cordifolia (Guduchi): a miraculous herb countering various diseases/disorders and its Immunomodulatory effects. Recent Pat Endocr Metab Immune Drug Discov 10(2):96–111. https://doi.org/10.2174/1872214811666170301105101
Dixit R, Malaviya D, Pandiyan K, Singh UB, Sahu A, Shukla R, Paul D (2015) Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability 72:2189–2212. https://doi.org/10.3390/su7022189
Dubey A, Shiwani S (2012) Adsorption of lead using a new green material obtained from Portulaca plant. Int J Environ Sci Technol 9:15–20. https://doi.org/10.1007/s13762-011-0012-8
Duruibe JO, Ogwuegbu MOC, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. In J Phys Sci 25:112–11. https://doi.org/10.5897/IJPS.9000289
Edelstein M, Ben-Hur M (2018) Heavy metals and metalloids: sources, risks and strategies to reduce their accumulation in horticultural crops. Sci Hortic 234:431–444. https://doi.org/10.1016/j.scienta.2017.12.039
El-Kady AA, Abdel-Wahhab MA (2018) Occurrence of trace metals in foodstuffs and their health impact. Trends Food Sci Technol 75:36–45. https://doi.org/10.1016/j.tifs.2018.03.001
Ent AVD, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 3621–2:319–334. https://doi.org/10.1007/s11104-012-1287-3
Farrell M, Jones DL (2010) Use of composts in the remediation of heavy metal contaminated soil. J. Hazard. Mater. 175, 575–582 soil using nano-crystallite hydroxyapatite. Procedia Environ Sci 18:657–665. https://doi.org/10.1016/j.jhazmat.2009.10.044
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5(2):47
Fu Z, Xi S (2020) The effects of heavy metals on human metabolism. Toxicol Mech Methods 303:167–176. https://doi.org/10.1080/15376516.2019.1701594
Gall JE, Boyd RS, Rajakaruna N (2015) Transfer of heavy metals through terrestrial food webs: a review. Environ Monit Assess 187:201. https://doi.org/10.1007/s10661-015-4436-3
Gebrekidan A, Weldegebriel Y, Hadera A, Bruggen BVD (2013) Toxicological assessment of heavy metals accumulated in vegetables and fruits grown in Ginfel river near Sheba Tannery, Tigray, Northern Ethiopia. Ecotoxicol Environ Saf 95:171–178. https://doi.org/10.1016/j.ecoenv.2013.05.035
Gedik K (2018) Bioaccessibility of heavy metals in rapa whelk Rapana venosa (Valenciennes, 1846): Assessing human health risk using an in vitro digestion model. Hum Ecol Risk Assess Int J 24(1):202–213. https://doi.org/10.1080/10807039.2017.1373329
Meselhy AG, Sharma S, Guo Z, Singh G, Yuan H, Tripathi RD, ... Dhankher OP (2021) Nanoscale sulfur improves plant growth and reduces arsenic toxicity and accumulation in rice (Oryza sativa L.). Environ Sci Technol 55(20): 13490–13503. https://doi.org/10.1021/acs.est.1c05495
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. Int J Environ Res Public Health 17(11):3782. https://doi.org/10.3390/ijerph17113782
Ghosal PS, Kattil KV, Yadav MK, Gupta AK (2018) Adsorptive removal of arsenic by novel iron/olivine composite: insights into preparation and adsorption process by response surface methodology and artificial neural network. J Environ Manag 209:176–187. https://doi.org/10.1016/j.jenvman.2017.12.040
Gomes MVT, de Souza RR, Teles VS, Mendes ÉA (2014) Phytoremediation of water contaminated with mercury using Typha domingensis in constructed wetland. Chemosphere 103:228–233. https://doi.org/10.1016/j.chemosphere.2013.11.071
Gonzalez B, Heijman SGJ, Rietveld LC, van Halem D (2019) Arsenic removal from geothermal influenced groundwater with low pressure NF pilot plant for drinking water production in Nicaraguan rural communities. Sci Total Environ 667:297–305. https://doi.org/10.1016/j.scitotenv.2019.02.222
Gonzalez-Raymat H, Liu G, Liriano C, Li Y, Yin Y, Shi J, Cai Y (2017) Elemental mercury: Its unique properties affect its behavior and fate in theenvironment. Environ Pollut 229:69–86. https://doi.org/10.1016/j.envpol.2017.04.101
Gupta N, Khan DK, Santra SC (2012) Heavy metal accumulation in vegetables grown in a long-term wastewater-irrigated agricultural land of tropical India. Environ Monit Assess 18411:6673–6682. https://doi.org/10.1007/s10661-011-2450-7
Hamid Y, Lin T, Muhammad Y, Bilal H, Afsheen Z, Muhammad ZA, Zhen-li H, Xiaoe Y (2019) Comparative efficacy of organic and inorganic amendments for cadmium and lead immobilization in contaminated soil under rice, wheat cropping system. Chemosphere 214:259–268. https://doi.org/10.1016/j.chemosphere.2018.09.113
Hassan SE, Hijri M, St-Arnaud M (2013) Effect of arbuscular mycorrhizal fungi on trace metal uptake by sunflower plants grown on cadmium contaminated soil. N Biotech 30:780–787. https://doi.org/10.1016/j.nbt.2013.07.002
He X, Xu M, Wei Q, Tang M, Guan L, Lou L, ... Xia Y (2020) Promotion of growth and phytoextraction of cadmium and lead in Solanum nigrum L. mediated by plant-growth-promoting rhizobacteria. Ecotoxicol Environ Saf 205: 111333. https://doi.org/10.1016/j.ecoenv.2020.111333
Herzig R, Nehnevajova E, Pfistner C, Schwitzguebel JP, Ricci A, Keller C (2014) Feasibility of labile Zn phytoextraction using enhanced tobacco and sunflower: results of five-and one-year field-scale experiments in Switzerland. Int J Phytoremediation 16(7–8):735–754. https://doi.org/10.1080/15226514.2013.856846
Horsfall M Jr, Ogban FE, Akporhonor EE (2006) Recovery of lead and cadmium ions from metal-loaded biomass of wild cocoyam Caladium bicolor using acidic, basic and neutral eluent solutions. Electron J Biotechnol 92:0–0. https://doi.org/10.2225/vol9-issue2-fulltext-9
Hu H, Jin Q, Kavan P (2014) A study of heavy metal pollution in China: Current status, pollution-control policies and countermeasures. Sustainability 6(9):5820–5838. https://doi.org/10.3390/su6095820
Huang Y, Yang JK, Keller AA (2014) Removal of arsenic and phosphate from aqueous solution by metal (hydr-) oxide coated sand. ACS Sustain Chem Eng 2(5):1128–1138. https://doi.org/10.1021/sc400484s
Hwang T, Neculita CM (2013) In situ immobilization of heavy metals in severely weathered tailings amended with food waste-based compost and zeolite. Water Air Soil Pollut 224:1388. https://doi.org/10.1007/s11270-012-1388-x
IARC International Agency for Research on Cancer (2012) A review of human carcinogens: arsenic, metals, fibres, and dusts. World Health Organization Press, Lyon
Google Scholar
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 72:60–72. https://doi.org/10.2478/intox-2014-0009
Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QM (2015) Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci 1612:29592–29630. https://doi.org/10.3390/ijms161226183
Jarma YA, Karaoğlu A, Tekin Ö, Baba A, Ökten HE, Tomaszewska B, Kabay N (2021) Assessment of different nanofiltration and reverse osmosis membranes for simultaneous removal of arsenic and boron from spent geothermal water. J Hazard Mater 405:124129. https://doi.org/10.1016/j.jhazmat.2020.124129
Jiang W, Liu D (2010) Pb-induced cellular defense system in the root meristematic cells of Allium sativum L. BMC Plant Biol 101:1–8. https://doi.org/10.1186/1471-2229-10-40
Joseph L, Jun BM, Flora JR, Park CM, Yoon Y (2019) Removal of heavy metals from water sources in the developing world using low-cost materials: a review. Chemosphere 229:142–159. https://doi.org/10.1016/j.chemosphere.2019.04.198
Khan I, Iqbal M, Ashraf MY, Ashraf MA, Ali S (2016) Organic chelants-mediated enhanced lead Pb uptake and accumulation is associated with higher activity of enzymatic antioxidants in spinach Spinacea oleracea L. J Hazard Mater 317:352–361. https://doi.org/10.1016/j.jhazmat.2016.06.007
Khan A, Khan S, Khan MA, Qamar Z, Waqas M (2015) The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: a review. Environ Sci Pollut Res 2218:13772–13799. https://doi.org/10.1007/s11356-015-4881-0
Kim KH, Kabir E, Jahan SA (2016) A review on the distribution of Hg in the environment and its human health impacts. J Hazard Mater 306:376–385. https://doi.org/10.1016/j.jhazmat.2015.11.031
Kumar A, Cabral-Pinto MMS, Chaturvedi AK, Shabnam AA, Subrahmanyam G, Mondal R, Yadav KK (2020a) Lead toxicity: health hazards, influence on food chain, and sustainable remediation approaches. Int J Environ Res Public Health. 17(7):2179. https://doi.org/10.3390/ijerph17072179
Kumar A, Singh PK, Srivastava S, Dwivedi S, Tripathi RD, Awasthi G, Gupta K, Ansari MI (2020b)A Comparative Study on Effect of Arsenic on Thiolic Ligandsand Phytochelatins in Contrasting Arsenic Accumulating RiceGenotypes. International Journal of Plant and Environment 6(02):110–7
Kumar R, Patel M, Singh P, Bundschuh J, Pittman CU Jr, Trakal L, Mohan D (2019a) Emerging technologies for arsenic removal from drinking water in rural and peri-urban areas: methods, experience from, and options for Latin America. Sci Total Environ 694:133427. https://doi.org/10.1016/j.scitotenv.2019.07.233
Kumar S, Prasad S, Yadav KK, Shrivastava M, Gupta N, Nagar S, Malav LC (2019b) Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches-A review. Environ Res 179:108792. https://doi.org/10.1016/j.envres.2019.108792
Kyzas GZ, Kostoglou M (2015) Swelling-adsorption interactions during mercury and nickel ions removal by chitosan derivatives. Sep Purif Technol 149:92–102. https://doi.org/10.1016/j.seppur.2015.05.024
Lara RH, Vazquez-Arenas J, Ramos-Sanchez G, Galvan M, Lartundo-Rojas L (2015) Experimental and theoretical analysis accounting for differences of pyrite and chalcopyrite oxidative behaviors for prospective environmental and bioleaching applications. J Phys Chem C 11932:18364–18379. https://doi.org/10.1021/acs.jpcc.5b05149
Lee WM, An YJ, Yoon H, Kweon HS (2008) Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water-insoluble nanoparticles. Environ Toxicol Chem: an International Journal 27(9):1915–1921. https://doi.org/10.1897/07-481.1
Li R, Wu H, Ding J, Fu W, Gan L, Li Y (2017) Mercury pollution in vegetables, grains and soils from areas surrounding coal-fired power plants. Sci Rep 71:1–9. https://doi.org/10.1038/srep46545
Li Z, Wu L, Liu H, Lan H, Qu J (2013) Improvement ofaqueous mercury adsorption on activated coke by thiolfunctionalization. Chem Eng J 228:925–934. https://doi.org/10.1016/j.cej.2013.05.063
Lim HS, Lim W, Hu JY, Ziegler A, Ong SL (2015) Comparison of filter media materials for heavy metal removal from urban stormwater runoff using biofiltration systems. J Environ Manag 147:24–33. https://doi.org/10.1016/j.jenvman.2014.04.042
Liu R (2011) In-situ lead remediation in a shoot-range soil using stabilized apatite nanoparticles. In: Proceedings of the 85th ACS Colloid and Surface Science Symposium, McGill University, Montreal, Canada. https://doi.org/10.1155/2012/461468
Lone AH, Lal EP, Thakur S, Ganie SA, Wani MS, Khare A, Wani FA (2013) Accumulation of heavy metals on soil and vegetable crops grown on sewage and tube well water irrigation. Sci Res Essays 844:2187–2193. https://doi.org/10.5897/SRE2013.5636
Lv Y, Huang S, Huang G, Liu Y, Yang G, Lin C, Liu M (2020) Remediation of organic arsenic contaminants with heterogeneous Fenton process mediated by SiO 2-coated nano zero-valent iron. Environ Sci Pollut Res 27(11):12017–12029. https://doi.org/10.1007/s11356-020-07808-2
Ma X, Geiser-Lee J, Deng Y, Kolmakov A (2010) Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ 408(16):3053–3061. https://doi.org/10.1016/j.scitotenv.2010.03.031
Ma C, Hao Y, Zhao J, Zuverza-Mena N, Meselhy AG, Dhankher OP, Xing B (2021) Graphitic carbon nitride (C3N4) reduces cadmium and arsenic phytotoxicity and accumulation in rice (Oryza sativa L.). Nanomaterials. 11(4):839. https://doi.org/10.3390/nano11040839
Maitlo HA, Kim JH, Kim KH, Park JY, Khan A (2019) Metal-air fuel cell electrocoagulation techniques for the treatment of arsenic in water. J Clean Prod 207:67–84. https://doi.org/10.1016/j.jclepro.2018.09.232
Mani S, Bharagava RN (2016) Exposure to Crystal Violet, its toxic, genotoxic and carcinogenic effects on environmental and its degradation and detoxification for environmental safety. Rev Environ Contam Toxicol 237:71–104. https://doi.org/10.1007/978-3-319-23573-8_4
Manwani S, Vanisree CR, Jaiman V, Awasthi KK, Yadav CS, Sankhla MS, Pandit PP, Awasthi G (2022a) Heavy Metal Contamination in Vegetables and Their Toxic Effects on Human Health. Sustainable Crop Production: Recent Advances 181
Manwani S, Sharma A, Bhoot N, Awasthi A, Awasthi G (2022b) Comparative analysis of different chemical and biological adsorbents for the reduction of arsenic and mercury from vegetable species in the Sanganer, Jaipur. Materials Today: Proceedings
Manwani S, Bhoot N, Pandey H, Awasthi G (2022c) Mitigation of lead and Cadmium from vegetable crops through different biochemical adsorbent treatments at Sanganer industrial area, Jaipur. Materials Today: Proceedings
Martorell I, Perelló G, Martí-Cid R, Llobet JM, Castell V, Domingo JL (2011) Human exposure to arsenic, cadmium, mercury, and lead from foods in Catalonia, Spain: temporal trend. Biol Trace Elem Res 142:309–322. https://doi.org/10.1007/s12011-010-8787-x
Matović V, Buha A, Ðukić-Ćosić D, Bulat Z (2015) Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem Toxicol 78:130–140. https://doi.org/10.1016/j.fct.2015.02.011
McLaughlin MJ, Smolders E, Degryse F, Rietra R (2011) Uptake of metals from soil into vegetables. Dealing with contaminated sites. Springer, Dordrecht, pp 325–367. https://doi.org/10.1007/978-90-481-9757-6_8
Milner MJ, Kochian LV (2008) Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann Bot 1021:3–13. https://doi.org/10.1093/aob/mcn063
Minnikova TV, Denisova TV, Mandzhieva SS, Kolesnikov SI, Minkina TM, Chaplygin VA, Bauer TV (2017) Assessing the effect of heavy metals from the Novocherkassk power station emissions on the biological activity of soils in the adjacent areas. J Geochem Explor 174:70–78. https://doi.org/10.1016/j.gexplo.2016.06.007
Mishra BK, Dubey CS, Shukla DP, Bhattacharya P, Usham AL (2014) Concentration of arsenic by selected vegetables cultivated in the Yamuna flood plains YFP of Delhi, India. Environ Earth Sci 729:3281–3291. https://doi.org/10.1007/s12665-014-3232-7
Mishra S, Bharagava RN, More N, Yadav A, Zainith S, Mani S, Chowdhary P (2019) Heavy metal contamination: an alarming threat to environment and human health. Environmental biotechnology: For sustainable future. Springer, Singapore, pp 103–125. https://doi.org/10.1007/978-981-10-7284-0_5
Mishra S, Mattusch J, Wennrich R (2017) Accumulation and transformation of inorganic and organic arsenic in rice and role of thiol-complexation to restrict their translocation to shoot. Sci Rep 71:1–13. https://doi.org/10.1038/srep40522
Mohamed I, Bocar A, Ming L, Changxiu G, Peng C, Wei L, Qiaoyun H (2010) Fractionation of copper and cadmium and their binding with soil organic matter in a contaminated soil amended with organic materials. J Soils Sediments 10:973–982. https://doi.org/10.1007/s11368-010-0199-1
Mondal S, Bandopadhyay P, Dutta P (2018) Arsenic contamination in cropping systems under varying irrigation sources in the deltaic plain of India. Arch Agron Soil Sci 6412:1759–1767. https://doi.org/10.1080/03650340.2018.1453132
Montenegro AC, Ferreyroa GV, Parolo ME, Tudino MB, Lavado RS, Molina FV (2015) Copper speciation in soil: time evolution and effect of clay amendment. Water Air Soil Pollut 226:1–10. https://doi.org/10.1007/s11270-015-2569-1
Najafi M, Yousefi Y, Rafati AA (2012) Synthesis, characterization and adsorption studies of several heavy metal ions on amino functionalized silica nano hollow sphere and silica gel. Separ Purif Technol 852:193–205. https://doi.org/10.1016/j.seppur.2011.10.011
Neina D (2019) The role of soil pH in plant nutrition and soil remediation. Appl Environ Soil Sci 1-9 https://doi.org/10.1155/2019/5794869
Niu LQ, Jia P, Li SP, Kuang JL, He XX, Zhou WH, Liao B, Shu WS, Li JT (2015) Slash-and-char: an ancient agricultural technique holds new promise for management of soils contaminated by Cd, Pb and Zn. Environ Pollut 205:333–339. https://doi.org/10.1016/j.envpol.2015.06.017
Nogawa K, Sakurai M, Ishizaki M, Kido T, Nakagawa H, Suwazono Y (2017) Threshold limit values of the cadmium concentration in rice in the development of itai-itai disease using benchmark dose analysis. J Appl Toxicol 37(8):962–966. https://doi.org/10.1002/jat.3444
Nogueira TAR, Franco A, He Z, Braga VS, Firme LP, Abreu-Junior CH (2013) Short-term usage of sewage sludge as organic fertilizer to sugarcane in a tropical soil bears little threat of heavy metal contamination. J Environ Manage 114:168–177. https://doi.org/10.1016/j.jenvman.2012.09.012
O’Connor D, Peng T, Li G, Wang S, Duan L, Mulder J, Cornelissen G, Cheng Z, Yang S, Hou D (2018) Sulfur-modified rice husk biochar: a green method for the remediation of mercury contaminated soil. Sci Total Environ 621:819–826. https://doi.org/10.1016/j.scitotenv.2017.11.213
Pandey J, Pandey R, Shubhashish K (2009) Air-borne heavy metal contamination to dietary vegetables: a case study from India. Bull Environ Contam Toxicol 836:931–936. https://doi.org/10.1007/s00128-009-9879-1
Pazoki A, Kashani A, Ilkaei MN, Aghayari F, Rahmani M, Far MD, Adraki M (2014) Effects of PGPR and mycorrhiza on morphological traits and yield components of wheat under different lead levels. Int J Biosci 4:216–221. https://doi.org/10.12692/ijb/4.12.216-221
Peralta-Videa JR, Lopez ML, Narayan M, Saupe G, Gardea-Torresdey J (2009) The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. Int J Biochem Cell Biol 41(8–9):1665–1677. https://doi.org/10.1016/j.biocel.2009.03.005
Pogrzeba M, Krzyżak J, Rusinowski S, McCalmont JP, Jensen E (2019) Energy crop at heavy metal-contaminated arable land as an alternative for food and feed production: biomass quantity and quality. Plant Metallomics and Functional Omics. Springer, Cham, pp 1–21. https://doi.org/10.1007/978-3-030-19103-0_1
Pramod L, Gandhimathi R, Lavanya A, Ramesh ST, Nidheesh PV (2019) Heterogeneous Fenton process coupled with microfiltration for the treatment of water with higher arsenic content. Chem Eng Commun 207(12):1646–1657. https://doi.org/10.1080/00986445.2019.1674814
Rahman Z, Singh VP (2019) The relative impact of toxic heavy metals (THMs)(arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environ Monit Assess 191(7):1–21. https://doi.org/10.1007/s10661-019-7528-7
Rai PK (2012) An eco-sustainable green approach for heavy metals management: two case studies of developing industrial region. Environ Monit Assess 1841:421–448. https://doi.org/10.1007/s10661-011-1978-x
Rai PK, Lee J, Kailasa SK, Kwon EE, Tsang YF, Ok YS, Kim KH (2018) A critical review of ferrate VI-based remediation of soil and groundwater. Environ Res 160:420–448. https://doi.org/10.1016/j.envres.2017.10.016
Raj D, Kumar A, Maiti SK (2020) Mercury remediation potential of Brassica juncea L. Czern. for clean-up of flyash contaminated sites. Chemosphere. 248:125857. https://doi.org/10.1016/j.chemosphere.2020.125857
Raj D, Maiti SK (2019) Bioaccumulation of potentially toxic elements in tree and vegetable species with associated health and ecological risks: a case study from a thermal power plant, Chandrapura, India. Rendiconti Lincei Scienze Fisiche e Naturali 303:649–665. https://doi.org/10.1007/s12210-019-00831-7
Rice KM, Walker EM Jr, Wu M, Gillette C, Blough ER (2014) Environmental mercury and its toxic effects. J Prev Med Public Health. 472:74. https://doi.org/10.3961/2Fjpmph.2014.47.2.74
Roy S, Edwards MA (2019) Preventing another lead Pb in drinking water crisis: lessons from the Washington DC and Flint MI contamination events. Curr Opin Environ Sci Health 7:34–44. https://doi.org/10.1016/j.coesh.2018.10.002
Saif S, Khan MS, Zaidi A, Rizvi A, Shahid M (2017) Metal toxicity to certain vegetables and bioremediation of metal-polluted soils. Microbial Strategies for Vegetable Production. Springer, Cham, pp 167–196. https://doi.org/10.1007/978-3-319-54401-4_8
Samal AC, Kar S, Bhattacharya P, Santra SC (2011) Human exposure to arsenic through foodstuffs cultivated using arsenic contaminated groundwater in areas of West Bengal, India. J Environ Sci Health Part A 4611:1259–1265. https://doi.org/10.1080/10934529.2011.598810
Sarwar N, Imran M, Shaheen MR, Ishaque W, Kamran MA, Matloob A, Rehim A, Hussain S (2017) Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere 171:710–721. https://doi.org/10.1016/j.chemosphere.2016.12.116
Sato A, Hiroyuki T, Wataru O, Eiji N, Masaharu M (2010) Reduction of cadmium uptake in spinach Spinacia oleracea L. by soil amendment with animal waste compost.J. Hazard Mater 181:298–304. https://doi.org/10.1016/j.jhazmat.2010.05.011
Schiewer S, Patil SB (2008) Pectin-rich fruit wastes as biosorbents for heavy metal removal: equilibrium and Kinetics. Bioresour Technol 996:1896–1903. https://doi.org/10.1016/j.biortech.2007.03.060
Shakoor M, Niazi NK, Irshad B, Murtaza G, Kunhikrishnan A, Seshadri B, Shahid M, Ali S, Bolan S, Yong N, Muhammad Abid SO, Ali F (2016) Remediation of arsenic contaminated water using agricultural wastes as biosorbents. Crit Rev Environ Sci Technol 46:467–499. https://doi.org/10.1080/10643389.2015.1109910
Shanab S, Essa A, Shalaby E (2012) Bioremoval capacity of three heavy metals by some microalgae species Egyptian Isolates. Plant Signal Behav 7:392–399. https://doi.org/10.4161/psb.19173
Sharma RK, Agrawal M, Marshall F (2007) Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol Environ Saf 662:258–266. https://doi.org/10.1016/j.ecoenv.2005.11.007
Sharma S, Prasad FM (2010) Accumulation of lead and cadmium in soil and vegetable crops along major highways in Agra (India). E-J Chem 7(4):1174–1183. https://doi.org/10.1155/2010/678589
Sheetal KR, Singh SD, Anand A, Prasad S (2016) Heavy metal accumulation and effects on growth, biomass and physiological processes in mustard, Indian. J Plant Physiol 212:219–223. https://doi.org/10.1007/s40502-016-0221-8
Shekar CC, Sammaiah D, Shasthree T, Reddy KJ (2011) Effect of mercury on tomato growth and yield attributes. Int J Pharm Bio Sci 22:358–364
Sikka R, Nayyar V (2012) Cadmium accumulation and its effects on uptake of micronutrients in Indian mustard [Brassica juncea L. czern.] grown in a loamy sand soil artificially contaminated with cadmium. Commun Soil Sci Plant Anal 434:672–688. https://doi.org/10.1080/00103624.2012.644007
Singh AP, Dixit G, Mishra S, Dwivedi S, Tiwari M, Mallick S, Tripathi RD (2015) Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front Plant Sci 6:340. https://doi.org/10.3389/fpls.2015.00340
Singh T, Singh DK (2017) Phytoremediation of organochlorine pesticides: Concept, method, and recent developments. Int J Phytorem 199:834–843. https://doi.org/10.1080/15226514.2017.1290579
Suman J, Uhlik O, Viktorova J, Macek T (2018) Phytoextraction of heavy metals: a promising tool for clean-up of polluted environment?. Front Plant Sci 1476. https://doi.org/10.3389/fpls.2018.01476
Sonu K, Yadav IC, Kumar A, Devi NL (2019) Dataset on assessment of heavy metals contamination in multi-environmental samples from Patna, India. Data in Brief 25:104079
Soghoian S, Sinert R (2008) Toxicity, heavy metals. http://emedicine.medscape.com/article/814960-overview , Accessed date: February 2019
Sud D, Mahajan G, Kaur MP (2008) Agricultural waste material as potential adsorbent for sequestering heavy metals ions from aqueous solutions: a review. Bioresour Technol 9914:6017–6027. https://doi.org/10.1016/j.biortech.2007.11.064
Sun HJ, Xiang P, Luo J, Hong H, Lin H, Li HB, Ma LQ (2016) Mechanisms of arsenic disruption on gonadal, adrenal and thyroid endocrine systems in humans: a review. Environ Int 95:61–68. https://doi.org/10.1016/j.envint.2016.07.020
Sun Y, Li Y, Xu Y, Liang X, Wang L (2015) In situ stabilization remediation of cadmium Cd and lead Pb co-contaminated paddy soil using bentonite. Appl Clay Sci 105–106:200–206. https://doi.org/10.1016/j.clay.2014.12.031
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Molecular, clinical and environmental toxicology. Springer, Basel, pp 133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Teng Z, Shao W, Zhang K, Yu F, Huo Y, Li M (2020) Enhanced passivation of lead with immobilized phosphate solubilizing bacteria beads loaded with biochar/nanoscale zero valent iron composite. J Hazard Mater 384:121505
Thévenod F,Lee WK (2013) Toxicology of cadmium and its damage to mammalian organs. Cadmium: from toxicity to essentiality, pp.415–490. https://doi.org/10.1007/978-94-007-5179-8_14
Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Maathuis FJ (2007) Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol 254:158–165. https://doi.org/10.1016/j.tibtech.2007.02.003
Ungureanu G, Santos S, Boaventura R, Botelho C (2015) Arsenic and antimony in water and wastewater: overview of removal techniques with special reference to latest advances in adsorption. J Environ Manag 151:326–342. https://doi.org/10.1016/j.jenvman.2014.12.051
Upadhyay MK, Shukla A, Yadav P, Srivastava S (2019) A review of arsenic in crops, vegetables, animals and food products. Food Chem 276:608–618. https://doi.org/10.1016/j.foodchem.2018.10.069
Usman M, Byrne JM, Chaudhary A, Orsetti S, Hanna K, Ruby C, Kappler A, Haderlein SB (2018) Magnetite and green rust: synthesis, properties, and environmental applications of mixed-valent Iron minerals. Chem Rev 118:3251–3304. https://doi.org/10.1021/acs.chemrev.7b00224
Vanisree CR, Sankhla MS, Singh P, Jadhav EB, Verma RK, Awasthi KK, Awasthi G, Nagar V (2022) Heavy metal contamination of food crops: Transportation via food chain, human consumption, toxicity and management strategies
Van Maele-Fabry G, Lombaert N, Lison D (2016) Dietary exposure to cadmium and risk of breast cancer in postmenopausal women: a systematic review and meta-analysis. Environ Int 86:1–13. https://doi.org/10.1016/j.envint.2015.10.003
Vats S, Gupta N, Bhargava P (2019) Vulnerability of soil micro biota towards natural and anthropogenic induced changes and loss of pedospheric functionality. Mycorrhizosphere and Pedogenesis. Springer, Singapore, pp 191–205. https://doi.org/10.1007/978-981-13-6480-8_12
Wang FY, Ling W, Zhao YS, You JL, Zhi MS (2012) Effects of AM inoculation and organic amendment, alone or in combination, on growth, P nutrition, and heavy-metal uptake of tobacco in Pb-Cd-contaminated soil. J Plant Growth Regul 31:549–559. https://doi.org/10.1007/s00344-012-9265-9
Wang Q, Chen L, He LY, Sheng XF (2016) Increased biomass and reduced heavy metal accumulation of edible tissues of vegetable crops in the presence of plant growth-promoting Neorhizobium huautlense T1–17 and biochar. Agr Ecosyst Environ 228:9–18. https://doi.org/10.1016/j.agee.2016.05.006
Wang Q, Zhang WJ, He LY, Sheng XF (2018) Increased biomass and quality and reduced heavy metal accumulation of edible tissues of vegetables in the presence of Cd-tolerant and immobilizing Bacillus megaterium H3. Ecotoxicol Environ Saf 148:269–274. https://doi.org/10.1016/j.ecoenv.2017.10.036
WHO/FAO. (2007) Joint FAO/WHO Food Standard Programme Codex Alimentarius Commission 13th Session. Report of the Thirty Eight Session of the Codex Committee on Food Hygiene. Houston, United States of America, ALINORM 07/30/13
Xiao R et al (2017) Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi, China. Ecotoxicol Environ Saf 141:17–24. https://doi.org/10.1016/j.ecoenv.2017.03.002
Xiu-Zhen H, Zhou DM, Li DD, Jiang P (2012) Growth, cadmium and zinc accumulation of ornamental sunflower Helianthus annuus L. in contaminated soil with different amendments. Pedosphere. 22(5):631–639. https://doi.org/10.1016/S1002-0160(12)60048-4
Xue W, Huang D, Zeng G, Wan J, Zhang C, Xu R, Deng R (2018) Nanoscale zero-valent iron coated with rhamnolipid as an effective stabilizer for immobilization of Cd and Pb in river sediments. J Hazard Mater 341:381–389. https://doi.org/10.1016/j.jhazmat.2017.06.028
Yan A, Wang Y, Tan SN, Mohd Yusof ML, Ghosh S, Chen Z (2020) Phytoremediation: a promising approach for revegetation of heavy metal-polluted land. Front Plant Sci 11:359. https://doi.org/10.3389/fpls.2020.00359
Yang Y, Chang AC, Wang M, Chen W, Peng C (2018) Assessing cadmium exposure risks of vegetables with plant uptake factor and soil property. Environ Pollut 238:263–269. https://doi.org/10.1016/j.envpol.2018.02.059
Yousaf B, Liu G, Abbas Q, Ullah H, Wang R, Zia-ur-Rehman M, Niu Z (2018) Comparative effects of biochar-nanosheets and conventional organic-amendments on health risks abatement of potentially toxic elements via consumption of wheat grown on industrially contaminated-soil. Chemosphere 192:161–170. https://doi.org/10.1016/j.chemosphere.2017.10.137
Zeng P, Guo Z, Xiao X, Zhou H, Gu J, Liao B (2021) Tolerance capacities of Broussonetia papyrifera to heavy metal (loid) s and its phytoremediation potential of the contaminated soil. Int J Phytoremediation 1-10 https://doi.org/10.1080/15226514.2021.1958746
Zeng H, Yu Y, Wang F, Zhang J, Li D (2020) Arsenic V removal by granular adsorbents made from water treatment residuals materials and chitosan. Colloid Surf Physicochem Eng Aspect 585:124036. https://doi.org/10.1016/j.colsurfa.2019.124036
Zhang G, Qu J, Liu H, Cooper AT, Wu R (2007) CuFe2O4/activated carbon composite: a novel magnetic adsorbent for the removal of acid orange II and catalytic regeneration. Chemosphere 686:1058–1066. https://doi.org/10.1016/j.chemosphere.2007.01.081
Zhao L, Li T, Zhang X, Chen G, Zheng Z, Yu H (2016a) Pb uptake and phytostabilization potential of the mining ecotype of Athyrium wardii Hook. grown in Pb-contaminated soil. Clean: Soil, Air, Water 44:1184–1190. https://doi.org/10.1002/clen.201400870
Zhao L, Huang Y, Hu J, Zhou H, Adeleye AS, Keller AA (2016b) 1H NMR and GC-MS based metabolomics reveal defense and detoxification mechanism of cucumber plant under nano-Cu stress. Environ Sci Technol 50(4):2000–2010. https://doi.org/10.1021/acs.est.5b05011
Zhou H, Yang WT, Zhou X, Liu L, Gu JF, Wang WL, Liao BH (2016) Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. Int J Environ Res Public Health 133:289. https://doi.org/10.3390/ijerph13030289
Download references
Acknowledgements
We are thankful to Department of Life Sciences, Vivekananda Global University for providing necessary infrastructure to conduct the research.
Author information
Authors and affiliations.
Department of Life Sciences, Vivekananda Global University, Jaipur, Rajasthan, 303012, India
Seema Manwani, Pooja Devi, Chandra Shekhar Yadav, Kumud Kant Awasthi & Garima Awasthi
Department of Zoology, Delhi University, Delhi, 110007, India
Tanvi Singh
Central Laboratory, Rajasthan State Pollution Control Board, Jaipur, Rajasthan, 302004, India
Narain Bhoot
School of Forensic Science, National Forensic Science University, Gandhinagar, 382007, India
Chandra Shekhar Yadav
You can also search for this author in PubMed Google Scholar
Contributions
Writing, literature search, original draft, and editing were done by Seema Manwani and Pooja Devi. Conceptualization, visualization, review, editing, and formatting were performed by Tanvi Singh, Chandra Shekhar Yadav, Kumud Kant Awasthi, Narain Bhoot, and Garima Awasthi. All authors contributed to the study conception and design.
Corresponding author
Correspondence to Garima Awasthi .
Ethics declarations
Ethical approval, consent to participate, consent to publish, conflict of interest.
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Manwani, S., Devi, P., Singh, T. et al. Heavy metals in vegetables: a review of status, human health concerns, and management options. Environ Sci Pollut Res 30 , 71940–71956 (2023). https://doi.org/10.1007/s11356-022-22210-w
Download citation
Received : 14 March 2022
Accepted : 21 July 2022
Published : 03 August 2022
Issue Date : June 2023
DOI : https://doi.org/10.1007/s11356-022-22210-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Contamination
- Health impact and management
- Heavy metals
- Find a journal
- Publish with us
- Track your research

COMMENTS
metals in vegetables and their impact on the nutrient quality of vegetables: A review, Journal of Plant Nutrition, DOI: 10.1080/01904167.2018.1462382 To link to this article: https://doi.or g/10. ...
In this review, the problem of heavy metal contamination in vegetables is discussed along with the prospective management strategies like soil amendments, application of bioadsorbents, membrane ...
The article reviewed the existing understanding of how those released toxic heavy metals penetrate the food chain, biomagnify into cells when they are consumed as vegetables, and cause potentially ...
For sustainable global growth, food security is a prime concern issue, both quantitatively and qualitatively. Adverse effects on crop quality from contaminants like heavy metals have affected food security and human health. Vegetables comprise the essential and nutritious part of the human diet as they contain a lot of health-promoting minerals and vitamins. However, the inadvertent excess ...
For sustainable global growth, food security is a prime concern issue, both quantitatively and qualitatively. Adverse effects on crop quality from contaminants like heavy metals have affected food security and human health. Vegetables comprise the essential and nutritious part of the human diet as t …
Heavy metals can be readily taken up by vegetable roots, and can be accumulated at high levels in the edible parts of vegetables, even heavy metal in soil at low levels [16,17]. In many countries and regions, vegetables are exposed to heavy metals by various means, thus vegetable consumption can cause adverse health effects.
A literature review shows that the most popular indices are daily intake of metals (DIM) and health risk index (HRI) [9,11,13,14,15,16]. Daily intake of metals can help assess the relative phyto-availability of metals and is proportional to metal concentrations in the edible parts of vegetables as well as the consumed amounts of those ...
This review emphasizes the role of toxic metal remediation approaches due to their broad sustainability and applicability. The rapid developmental processes can incorporate a large quantity of hazardous and unseen heavy metals in all the segments of the environment, including soil, water, air and plants. ... The method used in the literature ...
This article reviews the presence of heavy metals in different vegetables, their mechanism of absorption, impact of heavy metals on physiology, and nutrient reduction and associated impact on humans with emphasis on pregnant women based on the existing scientific literature. However, a limited number of studies was found in the data base that ...
In this review, the problem of heavy metal contamination in vegetables is discussed along with the prospective management strategies like soil amendments, application of bioadsorbents, membrane ltration, bioremediation, and nanoremediation. Keywords Contamination · Health impact and management · Heavy metals · Vegetables Introduction