50+ Clinical Trials with Compensation Near You
Home / Clinical Trials / 50+ Clinical Trials with Compensation Near You
Clinical trials with compensation are a great opportunity to get involved in important medical research. A clinical trial is held to gather data on the potential of a novel treatment to address a specific illness. A clinical trial often focuses on people with a certain chronic condition or genetic risk factors.
A clinical study with compensation focuses on paying individual for participating in their trials. This helps study teams to more quickly drive interest around a trial, and also helps to ensure that clinical trial participants subsist through the entire trial. Some clinical trials with compensation also recruit healthy volunteers, those with no specific diagnosis or special medical needs. Which volunteers are needed for which study depends on the study’s goals. Nobody qualifies for every study, but through effective upfront communication with the study team most participants can quickly learn whether or not they are a good fit for an observational study or investigational treatment.
Compensation for research studies is a hot topic for both participants and organizers. Not all clinical trials with compensation fit everyone, but the right trial can help participants defray their costs and earn some money – anywhere from just $10 for a single visit to multiple thousands for longer studies.
Each clinical trial responds to diverse needs, so there’s no way of knowing precisely how much any given study will offer in compensation. With that said, there are a few basic facts you should know as you do research and decide whether participating in a trial focused on an investigational drug or treatment plan is the right fit for you.

Find Trials With Compensation
Register for trial updates, clinical trials for healthy volunteers with compensation, how much can volunteers make in clinical trials with compensation.
Compensation for clinical trials varies widely. It can range anywhere from below $100 to thousands of dollars for qualified participants. Compensation generally scales with the length and complexity of the trial, with individuals participating in the entire study maximizing their compensation. The shorter a clinical research study, the lower the amount of compensation offered, just as with research studies in other fields.
Can You Get Expenses Paid for in a Clinical Trial?
When you are cleared to participate in a research study, you will usually have any direct costs related to the study covered by the presiding company or organization. For example, you will not need to pay for a treatment given to you as part of a research study, and it does not matter if you have insurance.
Depending on the structure of the study, you may or may not qualify to have certain indirect expenses paid for. The most common indirect expense is travel. If you need to travel a long way from your home to a laboratory facility, you can often submit receipts for gas or other expenses for reimbursement.
When Do You Receive Clinical Trial Compensation?
Reimbursements are generally processed soon after they are received. On the other hand, if you’ve been offered a flat sum for your participation in the study, you will usually only receive it at a certain point in the study or after specific conditions are met.
These “conditions” usually relate to the length of the study. For example, a study that’s intended to run for a year might compensate volunteers every three months. This recognizes the fact that even some of the volunteers who qualify will not necessarily stay with the study for its entire duration.
Which Clinical Trials Offer the Most Study Compensation?
There’s no way of knowing exactly how much any given trial will provide in compensation until you contact the study sponsor. In order to make sure that you are a good fit for the trial, there will be a thorough screening process that makes sure you are a good fit, pass the exclusion criteria, and will complete the trial. As a general guideline, though, more complex clinical trials provide more compensation.
Many clinical trials have only a few basic requirements. As long as you meet the exclusion criteria, all you need to do is take the medical treatments as directed and occasionally get blood tests or other laboratory tests. But sometimes, researchers will need more information from you or will be in contact more frequently with the study physician or research team.
For instance, you might be asked to maintain a “clinical trial diary” that gives greater insight into how a condition responds at different times after treatment. This is an example of something the study research staff might figure into their calculations when determining compensation.
Is Compensation for Clinical Trial Participation Based on the Study’s Outcome?
In a word, no. A clinical trial will not offer its volunteers more or less money based on whether or not the research was successful, but rather fully participating in studies should aware you with the full compensation package. In fact, it might be months or even years before anyone can say with full certainty whether a study had the results the sponsor hoped for.
While you’re never responsible for the outcome of a study, your level of participation is definitely a factor. If you accidentally fail to follow instructions (on treatment dosage or timing, for example) it potentially invalidates your data, which may preclude compensation.
How Do I Find Out How Much Compensation a Trial Offers?
Most clinical trials with compensation advertise that compensation is available. However, a study sponsor might be legally limited in how much information they can publicize. Once it’s been verified that you match the required volunteer profile, you will receive written documentation of compensation and requirements. In some situations, an annual trial compensation package might be appropriate based on the types of interventions or medical treatments being tested.
Compensation for clinical trials makes it easier and more accessible to be a part of important medical research. Your decision to participate could help people all around the world that you will never meet, and help study drugs and treatment get out to market more quickly.
Are There Clinical Trials That Offer Compensation Near Me?
Most studies, especially ones that focus on healthy volunteers, have various locations and can even be ran in multiple countries. While technology is still being developed to allow participants to remotely participate in clinical trials, generally most study teams and study sponsors still require clinical trial participants to meet in-person based on the study design during the trial to check in on the progress of a treatment. Most major cities in the United States will offer some clinical trials that offer financial compensation and studies with locations near you.
Other Conditions
- Acute Aortic Dissection
- Ankylosing Spondylitis
- Alzheimer’s
- Auditory Loss and Deafness
- Autoimmune Hepatitis
- Bronchiectasis
- Breast Cancer
- Esophageal Cancer
- Lung Cancer
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer
- Compensation
- COPD Treatments
- Coronavirus
- Crohn’s Disease
- Diet And Nutrition
- Endometriosis
- Fibromyalgia
- Hepatitis B
- Hepatitis C
- Mindfulness
- Multiple Sclerosis
- Myasthenia Gravis
- Ophthalmology
- Osteoarthritis
- Parkinson’s Disease
- PBC and PSC
- Plant Based Diet
- Plastic Surgery
- Pulmonary Fibrosis
- Ulcerative Colitis
- Urinary Tract Infections
- Weight Loss
Trials With Compensation
Accepting Healthy Volunteers From Across the US.
Register for Updates
Receive notifications for trials that match your criteria.
Sign up to receive email notifications on clinical trials for individual conditions in your local area.
Tips for Compensating Research Participants
Researchers should consider the risk of coercion when developing a compensation plan to pay participants for human subjects research..

Compensation is a predetermined form of payment provided to research participants for their engagement in a research activity. Compensation can include travel reimbursement (e.g., a preloaded METROcard), electronic gift cards, and cash. A small compensation as an incentive for completion of a study is permitted so long as such incentive is not coercive. Teachers College Institutional Review Board (IRB) does not consider compensation a benefit.
Distribution of compensation may also include other departments (e.g., accounts payable) besides the IRB office. Researchers should consult the appropriate sources to determine the best way to distribute payment to study participants.
This article will cover the following topics related to participant compensation:
- How to disclose your plan to compensate participants for their involvement in a research study.
- The potential for coercion and how to determine a fair compensation amount.
- Guidance specific to certain study populations.
Disclosing a Plan to Compensate Participants
An IRB protocol submission is composed of the IRB Application Template and any documentation relevant to the study, such as consent documents, recruitment materials , or site permission forms (e.g., Informed Consent Form Template , Site Permission Template ). In the IRB application and on consent forms, researchers should detail their plans to compensate participants. The Office for Human Research Protections (OHRP) recommends researchers include “a detailed account of the terms of payment, including a description of the conditions under which a subject would receive partial or no payment (e.g., what will happen if he or she withdraws part way through the research or the investigator removes a subject from the study for medical or noncompliance reasons),” and that “payment [may] be prorated for the time of participation in the study rather than delayed until study completion because the latter could unduly influence a subject’s decision to exercise his or her right to withdraw at any time” ( HHS.gov, Attachment A ).
A compensation plan should include…
- An explanation of how participants will be compensated.
- (e.g., “Compensation will be prorated for completion of each study activity”).
- The amount and form of compensation.
- (e.g., $25 Amazon gift card, $30 cash, $10 preloaded METROcard, etc.).
- How the researcher will distribute the compensation to participants, including any identifiable information that may be collected during the process.
- (e.g., “Participants will be given the option to enter their email address at the end of the survey if they would like to receive compensation. Upon successful completion of the survey, the researcher will send a $25 Amazon gift card to the participant’s email. Participants’ contact information and survey data will be stored separately and their personally identifiable information will not be published or presented publicly.”)
- Circumstances under which participants may or may not be compensated.
- (e.g., “You may leave the interview at any time. Participants who complete 75% of the survey questions or more will receive a $20 gift card.” Alternatively, “You may leave the study at any time. All eligible participants, regardless of whether they leave the study early, will receive a $10 preloaded METROcard.”) .
- For studies that extend over the course of several sessions or days, consider prorating the compensation.
- (e.g., “You will complete three one-hour interviews over the course of 6 months. After each interview, you will receive a $20 gift card. After completing all three interviews, you will be given an additional $10 gift card. Your total compensation for this study is up to $70.”)
Potential for Coercion
“Influence is contextual, and undue influence is likely to depend on an individual’s situation” (HHS.gov, Attachment A ). Federal guidelines rarely provide precise standards for determining undue influence. As a result, TC IRB reviews each protocol submission on a case-by-case basis. Based on provisions set by the Office for Human Research Protections (OHRP) , IRBs make reasonable assessments “to minimize the likelihood of undue influence or coercion occurring. For example, IRBs may restrict levels of financial or nonfinancial incentives for participation and should carefully review the information to be disclosed to potential subjects to ensure that the incentives and how they will be provided are clearly described. Known benefits should be stated accurately but not exaggerated, and potential or uncertain benefits should be stated as such, with clear language indicating how much is known about the uncertainty or likelihood of these potential benefits” (HHS.gov, Attachment A ).
When determining whether compensation is coercive or causes undue influence, the IRB will weigh the participant qualifications against the intensity of tasks and time spent on study activities. Participant compensation is typically metered on average wages (salary based on profession) and time spent on tasks. Compensation that is more (or less) than this amount should be justified by the researcher.
The IRB will also consider how the researchers describe the compensation in their recruitment materials. In general, information about compensation should be secondary (in both content and formatting) to information about the study. Recruitment materials that format or highlight compensation as the main feature, or make compensation an inducement to participate in a study, are coercive and will be sent back to the researcher for revisions.
An acceptable recruitment flyer might read, “This study will examine the effects of sleep habits and sleep deprivation on the brain and body. You must be enrolled in a university program to be eligible to participate. This study is conducted by Dr. Anna Freud ( [email protected] ) at the Brain Lab, Teachers College, Columbia University (Protocol ID: 10-001). Participants will receive a physical exam and $100 for their participation.”
An unacceptable, or coercive, recruitment flyer might read, “Chance to get $100 and Free Medical Care just for talking about your Sleep Habits! Email [email protected] to get paid today!!”
Population Specific Guidances
The IRB will also consider whether the compensation is fitting for the population of interest. For example, youth should not be given monetary compensation. However, small gifts such as pens or erasers may be fitting for a short, school-based study.
New York City’s law states that NYC Department of Education teachers cannot receive compensation for participation in research studies . This means that TC researchers cannot give teachers monetary compensation for research conducted during class time or typical work hours. Researchers who would like to offer teachers compensation should conduct the study during the teachers’ personal time. Alternatively, if your research activities must fall during class time, consider providing teachers with non-monetary compensation (such as school supplies) or donations. For all studies, the IRB will make a decision on a case-by-case basis.
For more information on coercion and risk to participants, please review our guide to Understanding Potential Risks for Human Subjects Research . If you have specific questions about compensating participants, please contact the IRB at [email protected].
- NYC DOE IRB Information for Principals
- OHRP's Attachment A
- AMA Journal of Ethics: When Does the Amount We Pay Participants Become "Undue Influence"?
— Kailee Kodama Muscente, M.A.
Tags: Recruitment Consent New to IRB
Published Tuesday, Jul 6, 2021
Institutional Review Board
Address: Russell Hall, Room 13
* Phone: 212-678-4105 * Email: [email protected]
Appointments are available by request . Make sure to have your IRB protocol number (e.g., 19-011) available. If you are unable to access any of the downloadable resources, please contact OASID via email [email protected] .
Division of Research
Compensation for research participants.
- The Research Cycle
- Conduct Research
- Human Subjects Research at Brown
- HRPP/IRB Policies and Guidance
- Recruiting Research Participants
Compensating research participants is a way to thank them for contributing their time and recognize their willingness to take on some of the research-related risks of study activities. As compensation is an expression of gratitude by researchers for their participants, it is not a benefit of participation, and it must be fair and equitable. However, offering compensation is not required or expected.
Review of Compensation Plan
Brown’s Human Research Protection Program (HRPP) team, along with the Institutional Review Board (IRB), reviews and determines whether compensation is reasonable and if it unduly influences participation. All information concerning compensation — including the method, amount and schedule of payment — should be clearly stated when submitting study materials for HRPP/IRB review.
The HRPP team and IRB will take into account information from the community and culture as it evaluates the appropriateness of participant compensation.
Consent Forms for Research Participants
Compensation and Taxes
All payments and gifts to research participants are considered income by the Internal Revenue Service (IRS) and may be reportable to the IRS as taxable income. You are responsible for informing participants during the consent process that the payment they receive is taxable income and may be reportable to the IRS.
If a participant receives $600 or more from one or across a combination of research studies at Brown University within one calendar year, they will be taxed on this income. You are required to provide to Accounts Payable a participant’s legal name, address, Social Security number and the amount paid for participation. Brown uses this information to issue 1099 statements to study participants.
Accounts Payable Office
Reimbursement vs. Compensation
Reimbursement is not compensation for research participation. You may reimburse research participants (or their caregivers) for out-of-pocket expenses they have as a result of study enrollment, such as transportation, childcare, meals and similar expenses. Reimbursement payments are not reportable to the IRS as taxable income.
Offering reimbursement is not required or expected.
Compensation Methods at Brown
The Brown University’s Controller’s Office is responsible for ensuring the proper stewardship of the University’s financial resources. The HRPP team and IRB rely on the Controller’s Office to determine which forms of compensation comply with federal and state laws and University policy. Only these forms of compensation are available to investigators for human subjects research.
You are strongly encouraged to design your study using one of the compensation methods approved by the Controller’s Office, listed below. If you intend to use a method of compensation that is not approved, you will need to contact Accounts Payable for consultation and approval for its use before including the compensation in your study materials submitted for HRPP/IRB review.
Brown uses ClinCard to manage the reimbursement process for study participants. ClinCard is a participant payment system that makes it easier, faster and more secure to provide compensation to participants and to track payments through an online system. Studies that use the ClinCard as a means to provide monetary compensation to participants must include required language in the consent document and provide participants with IRB-approved FAQs about the system. Study staff may also be required to attend training on the use of the system.
If study participants wish to cash out their ClinCard balance at a bank teller, as a means to get cash without incurring a fee, it is recommended that they take a copy of the ClinCard Bank Instructions with them.
If you are interested in using the ClinCard in your study, please contact the Controller's Office. Additional guidance can be found on the office’s ClinCard page.
- Approved Language for ClinCard Consent
- ClinCard FAQs for Participants
- ClinCard Bank Instructions (PDF)
- Controller’s Office
- ClinCard Guidance
Cash and Gift Cards/Certificates
Cash payments and gift cards (plastic, paper or electronic) are common ways to compensate participants. Please review Brown’s Gift Card Policy for any requirements that may apply to your study.
Even if you will not collect identifying information about your participants, you should establish a way to track the cash payments and/or gift cards given to participants for reporting purposes to the Controller’s Office. For example, consider keeping a spreadsheet that links a unique identifying number from each gift card to an assigned study ID associated with a given participant.
Gift Card Policy
Unacceptable Compensation Methods
The Controller’s Office may determine that a compensation method does not meet Brown’s requirements for a number of reasons (i.e., it does not offer buyer or seller protection or there are no special authorizations, no offers of controls or reconciliation capability that would be acceptable to Brown or no ease of payments for the user). The determination of which compensation options are available for human subjects research is not a decision that is made by or can be overturned by either the HRPP team or the IRB.
If you would like to request that the Controller’s Office consider a method of compensation that is not already approved by Brown for use in human subjects research, please contact Accounts Payable at [email protected] or 401-863-2716.
At this time, the following compensation methods are specifically not allowed for human subjects research studies, as determined by the Controller’s Office:
- PayPal mobile payment service
- Venmo mobile payment service
Additional Guidance
Advertising compensation.
Your advertisement may state that participants will be compensated but should not emphasize the compensation by font (i.e., large or bold type) or design enhancements (i.e., exclamation marks, stars, subject/tag lines in electronic communications).
Prorated Compensation and Bonuses
Compensation for participation in research should not be contingent upon the participant completing the entire study but rather be prorated as the study progresses to insure voluntary participation. However, compensation of a small proportion as an incentive for completion of the study is acceptable providing that such an incentive does not constitute undue influence.
If a bonus is given at the completion of the study it should not be more than half of the total study compensation.
Sponsor Discounts
Compensation for participation in a clinical trial offered by a sponsor may not include a coupon good for a discount on the purchase price of the product once it has been approved for marketing.
- Whitepapers
- Ethics in Clinical Research
- Participant Recruitment
Compensating Participants in Clinical Research: Current Thinking

In clinical research studies, it is not uncommon for monetary compensation to be provided to research participants; as reimbursement for study-related expenses, as compensation for time and effort, and even as incentive payments to encourage enrollment. Sponsors, researchers and Institutional Review Boards (IRBs) are often wary about payments in research participation, citing concerns about coercion and undue influence, whether real or perceived, and have avoided payments that are “too high.”
But new research on how people make decisions about research participation, and new approaches to this question, bring a new perspective; are payments to participants actually too low? This paper explores this question, and whether we should, in fact, worry much less about restricting compensation for research participants.
Undue Influence and Coercion
“…the IRB must conclude that participation in any protocol it approves is reasonable (i.e., not unreasonable) for individuals in the target study population. This is not to say that no risk remains or that participation in research would be in the best interest of potential participants. Neither is required in order to avoid undue influence.” 1
At the foundation of the concerns about research participant payment are the issues of undue influence and coercion. These words are not clearly defined in research regulations or guidance, and are often used interchangeably when talking about participant payment, but they actually have very different meanings.
To coerce means to achieve something by using force or threat. Situations of true coercion are rare in clinical study recruitment situations. An example of coercion might be a physician who is seeing a patient at a free clinic who says, “If you don’t agree to be in my research study, you can’t come here for care anymore.” Payment offers, though, are not force, nor are they threats. Therefore, offers of payment for participation in research can never be coercive.
Influence is a different concept. Influence, in itself, is not a bad thing. Everyone makes decisions about what they do based on factors that influence them, and sometimes those factors are financial. While many of us really enjoy our jobs, if our employer told us that we wouldn’t get paid anymore, we’d probably stop showing up for work. The issue, then, is not influence, but undue influence. In legal terms, undue influence means that someone makes someone else behave in a way that is contrary to their interests. In research, we often describe undue influence in study recruitment as someone agreeing to take risks that were not reasonable, because they were influenced by other considerations (in this situation, by the offer of money).
But as discussed in the excellent recent paper, Paying Research Participants: The Outsized Influence of “Undue Influence” by Emily Largent and Holly Fernandez Lynch 1 , the possibility of unreasonable risks requires more consideration as well. In order for an IRB to approve a research study, the Board must ensure that the risks of the research are reasonable in relation to the anticipated benefits, for the target study population. If this is the case, for a research protocol that has been IRB-approved and a potential participant who is in the target study population, how can the offer of payment influence them to take risks that are unreasonable, when the risks have already been determined to be reasonable? With this argument, Largent and Lynch explain that the potential problem of undue influence in IRB-approved research is significantly overestimated, although possible in some very specific situations (for example, when potential participants are likely to deceive the researchers about their eligibility or when they have some unique characteristics outside the IRB’s purview). In an effort to reduce the occurrence of these situations—although they are already rare—we as a research community have erred on the side of caution in preventing payment or encouraging payment to be kept relatively low. However, underpaying for participation results in the possible exploitation of research participants, the overburdening of certain populations who are willing to accept low payments, and the scientific risks of failed studies due to under-enrollment.1 For minimal risk research, any concern at all about compensation is likely unnecessary, as the risks are so low that it would be very unlikely that any participant could be making a decision to take a risk that is unreasonable.
Types of Payments to Participants
Reimbursement of study-related expenses.
Reimbursement of expenses related to participation in research studies, whether provided by the sponsor or by the institution, should never be of ethical concern. While there are rare instances in which ethically-acceptable studies involve requiring participants to pay for study-required procedures or medications—most often in situations where diagnostic testing is being used for both clinical and research purposes—for the most part, research participation should be cost-neutral. Making research cost-neutral helps to ensure the principle of distributive justice, and that the risks and benefits of research participation are fairly distributed. If each research visit involves out-of-pocket expenses for gas, food during a long wait between scheduled blood draws, parking fees, and child care, then only those who can afford those expenses would be able to participate in the research. Coverage of expenses for airfare, overnight hotel stays, and other long-distance travel for research participation used to prompt additional ethical concerns based on the higher amounts of money involved. Comfort for these practices has grown over the last several years, in part based on studies in rare diseases and more specific patient populations, where the research is conducted at centers of excellence but potential subjects may be coming from other states or even other countries.
A number of different models for covering out-of-pocket expenses are acceptable including collection of receipts and reimbursement in cash or check; vouchers for taxis, parking or meals; pre-funded debit cards; or providing a per-diem amount based on average and expected expenses. Comfort has also grown with using third party vendors such as Uber and Lyft to bring participants to study visits, with direct billing to the sponsor.
Compensation for Time and Effort
Studies which include compensation for the time and effort of research participants should make an effort to consider the actual time spent on the study, including study visits, tasks outside study visits (completing surveys or diaries), and even travel time to clinical sites, keeping in mind that participants may be missing work in order to complete the study requirements. Payment amounts should be high enough so that they do not take advantage of populations with lower income; proposed payment amounts are sometimes based on local minimum wages, which provides a handy benchmark, but basing study payment on a low wage does have the effect that anyone who makes more than that wage will be losing money if they miss work for study commitments.
There are a number of models that have been proposed for the compensation of research participants, including a wage-payment model, and payment based on market forces and supply and demand. 2
Incentive Payments
Some study plans include, either explicitly or implicitly, the payment to potential participants in a manner or at a rate that is intended to persuade them to participate in the research study, above what might be considered compensation for time or effort. For example, one study offered to pay the costs of elective plastic surgery for which the patients were already scheduled—several thousand dollars—if the patients agreed to participate in a 24-hour-long post-operative study comparing a new pain medication to the standard medication. Another study offered access to services (consultation with personal coaches) and gifts up to a value of approximately $6000 for participation in a study that required the completion of a survey every three months for a year. While the initial reaction to the amounts of money involved is usually caution, if we go back to the discussion of undue influence earlier in the paper, is there truly a valid concern? In both cases, an IRB had determined that the risks of the research were reasonable in relation to the potential benefits; in the second example, the risks were minimal. If the sponsor is willing to pay a certain amount of money to ensure that they were able to enroll the study with the necessary number of participants and in a reasonable amount of time, these types of payments should be acceptable.
Efforts to protect research participants from undue influence, and researchers and sponsors from perceptions of trying to use undue influence, have long been a major concern for IRBs. However, the true risk of undue influence is significantly lower than has often been assumed, when considering research that has been IRB-approved and for which the risks are considered to be reasonable. Instead, parties involved in research should consider whether payments to research participants are sometimes too low.
- Largent E and Lynch HF. Paying research participants: The outsized influence of “undue influence.” IRB: Ethics & Human Research 2017;39(4):1-9.
- Grady C. Payment of clinical research subjects. Journal of Clinical Investigation. 2005;115(7):1681-1687. doi:10.1172/JCI25694.
Don't trust your study to just anyone.
WCG's IRB experts are standing by to handle your study with the utmost urgency and care. Contact us today to find out the WCG difference!
Event Details
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Study participants incentives, compensation and reimbursement in resource-constrained settings
Takafira mduluza, nicholas midzi, donold duruza, paul ndebele.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Selected articles from the 3rd Ethics, Human Rights and Medical Law Conference (3rd EHRML)
Sylvester Chima, Takafira Mduluza and Julius Kipkemboi
Publication of this supplement has been funded by the College of Health Sciences and the Research Office at the University of Kwazulu-Natal. Articles originate from the 3rd EHRML conference, which was organized by Informa Life Sciences Exhibitions. The articles have undergone the journal's standard peer review process for supplements. The Supplement Editors declare that they have no competing interests.
7-9 May 2013
3rd Ethics, Human Rights and Medical Law Conference, Africa Health Congress 2013
Johannesburg, South Africa
Collection date 2013.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Introduction
Controversies still exists within the research fraternity on the form and level of incentives, compensation and reimbursement to study participants in resource-constrained settings. While most research activities contribute significantly to advancement of mankind, little has been considered in rewarding directly the research participants from resource-constrained areas.
A study was conducted in Zimbabwe to investigate views and expectations of various stakeholders on study participation incentives, compensation and reimbursement issues. Data was collected using various methods including a survey of about 1,008 parents/guardians of school children participating in various immunological cohort studies and parasitology surveys. Community advisory boards (CABs) at 9 of the sites were also consulted. Further, information was gathered during discussions held at a basic research ethics training workshop. The workshop had 45 participants that including 40 seasoned Zimbabwean researchers and 5 international research collaborators.
About 90% (907) of the study participants and guardians expected compensation of reasonable value, in view of the researchers' value and comparison to other sites regardless of economic status of the community. During discussion with researchers at a basic ethics training workshop, about 80% (32) believed that decisions on level of compensation should be determined by the local research ethics committees. While, the few international research collaborators were of the opinion that compensation should be in accordance with local guidelines, and incentives should be in line with funding. Both the CAB members and study participants expressed that there should be a clear distinction between study incentive and compensation accorded to individual and community expectations on benefits from studies. However, CABs expressed that their suggestions on incentives and compensation are often moderated by the regulatory authorities who cite fear of unknown concerns.
Overall, both personal and community benefits need to be considered colectively in future studies to be conducted in resource-constrained communities. There is projected fear that recruitment in future may be a challenge, now that almost every community, has somehow been reached and participated in some form of studies. A major concern on reimbursement, compensation or incentives should be internationally pegged regardless of different economic status of the individuals or communities where the study is to be conducted.
Keywords: study participants, incentives, compensation, reimbursement, resource-constrained
Biomedical studies are well known to add scientific solutions to problems bedeviling mankind, animals and their environment [ 1 - 3 ]. Through conducting research activities, some benefits are also realized by individuals and communities worldwide. Research programs assist in finding out new ways for treatment, to solve some medical problems and to improve the health standards of living for humans [ 4 ]. However, with the benefits realized at multiple-levels, concern is raised where the subjects of the research activities, who are at the core of the programme, rarely obtain any visible individual benefits [ 5 ].
Research participants indulge in different study protocols for different reasons. Sometimes the main driving force is beyond the participants' control [ 6 ]. While currently, the international research foundations and organizations are struggling to rationalize participation, in an effort to tame the research jungle [ 7 , 8 ]. However, at individual levels there are a couple of questions that go unanswered for the participants who are right at the bottom of the planning and the research protocol hierarchy [ 5 , 6 ]. Some of the pertinent questions by the research participants that go unanswered include the following: i). What is my immediate benefit? ii). Who will benefit from this work being conducted? ii). Why are these people (researchers) using all the resources in my community and yet we have so many other problems? iii). Why my community and not that other community? iv). The researchers are here for only 2 years, then what next? v). These people are well off, better than anyone in our community, so they must be benefiting from the activities?
While researchers are well aware of the main goal(s) including on how to achieve these through data/sample gathering, a lot need to be understood on the study participants and their feelings. Further, it is reasonable not to assume but to become part of the community and feel from within what the participants expect from taking part in studies and providing their biological specimens. Some progress has been achieved along these lines with moderate consideration on certain aspects that affect study participants in the form of repayment or reimbursement, which is replacement of what could have been lost or what could have been gained during the time the participant is involved in the research activities [ 9 - 13 ]. While there are other forms that have been coined into studies that include giving out incentives; that represents something that motivates or encourages participation. Incentives are rarely permitted by study regulators for reason still unclear to many researchers, especially when an incentive is considered as payment or a concession to stimulate greater participation, this is regularly not permitted by most in-country study regulatory agents and in certain instances by research groups [ 5 ]. Where the commonly applied consideration of benefits to study participation by researchers is compensation, representing something that makes up for an undesirable or unwelcome state of affair or this can be something, typically money awarded to someone as a recompense for loss, injury or suffering.
Most research activities involve an intertwined relationship between different players working together to achieve a common goal. In the case of simple investigative research emanating from a researcher, there are several regulatory authorities that may be responsible for giving approval for sample shipping or exchange, drug use and other commonly regulated activities [ 14 ]. Additionally, there could be some local authorities that are responsible for over sight within certain areas and research institutions. Key to the regulatory and monitoring biomedical research involving humans is the ministry of health and other ministries that deal with the public. In certain locations, there is political leadership that has control over access and running any research activities in their communities [ 5 ]. The biomedical researcher/investigator has to prepare documents that go through all the required local regulatory institutions and offices that include the ethics review board. The ethics review committees are believed to be representing the communities where research is to be conducted. The members are believed or expected to have the community at heart and to also have in-depth understanding of both traditional and cultural beliefs. In this hierarchy, the research community/participants are located right at the bottom or the receiving end. The concerns of the community or participants are assumed as represented by the structures within the areas and the regulatory authorities [ 5 , 14 ].
Controverses exists on the forms and levels of incentive, compensation and to lesser extent re-imbursement to study participants in resource-constrained settings. Inequity exists on addressing study participants' involvement in research studies according to economic status of the areas where the studies are conducted. Regulatory authorities need to reach a compromise, rather than dictate the level, type or amount. Currently, there is a huge demand for biomedical research or trial populations, especially in areas still developing and carrying the burden of diseases. Africa has abundant virgin testing grounds for new tools produced by biomedical and genomic revolution, and vaccines for several infections challenges. This is compelled by the great diseases burden with easy to reach sample size. Rarely are requests for conducting studies in the African populations denied due to the scarce and poor health facilities, hence communities and the leaders would be expecting access to some improved health tools and products through hosting of studies. In such areas where capacity is lacking, the biomedical research activities being conducted may seldom be observed and monitored closely by the regulatory authorities and even by CABs who may not understand the scientific implications of such studies. The participant is found to be lowly considered and rarely consulted during the design of the study protocols rather everything is assumed from consultations with ministries and regulatory authorities. Major concern is that the community settlements are often dispersed and individuals find it difficulty to have a common stand. While in recent years it has come to light that some sponsors/funders are ready to accommodate as long as proposed in the line of expenditure by the investigating team towards study participant incentives, reimbursement and even compensation, since the sponsors sometimes have commitment to the community by providing services including alleviating poverty. The whole stages of considering compensation or even rewarding study participants has never been appropriately debated taking into account the concerns of the communities. While application of ethical principles has no mathematical formula, this has to take into account various prevailing factors that include economic, social, cultural religious, civil protection systems and other relevant factors. These factors may lead to procedural differences, but the spirit of the principle remains the same.
Some unscrupulous international and local researchers take advantage of the poor and uncoordinated research systems in resource-constrained areas. Rarely would local authorities keep keen track of the activities, and such a system is bound for abuse. Further, due to rampant poverty coupled with ignorance (in a sense), and sometimes there is prevailing abundant human rights abuses; this entail disregard of community rights and respect [ 15 - 18 ]. Most research activities require monitoring and this decision must be reached at planning level in comparison to studies conducted in other sites in developing countries. The African health challenges expose research participants, and also including researchers and institutions to exploitation, coercion, enticement and inducement [ 5 , 19 - 22 ]. Resource constrained communities are generally deprived of most common attributes of a well-sustained and democratic societies [ 23 , 24 ]. The basic human rights are not observed and the individuals, probably through ignorance or due to none existence, do not have any recourse to law [ 23 ]. This aspect is not available in African communities and sometimes the poor participant living in resource-constrained community can get assistance from NGO who attempt to lobby on their behalf [ 25 , 26 ]. However, critically analyzing the situation reveals a couple of stages where such mishap may be avoided during the planning stages of the proposal. The sponsor/funder, investigator, regulatory authorities assume not aware of this infringement on the participants. Through activities in immunological studies and parasitology surveys conducted in Zimbabwean communities; a study was conducted to investigate views and expectations of various stakeholders on study participation incentives, compensation and reimbursement issues.
Study sites
Eleven communities from districts in Zimbabwe participated in the study as follows: Burma Valley, Charehwa in Mutoko, Magaya and Chigono in Murehwa, Goromonzi, Kariba, Magunje and Karoi in Hurungwe, Shamva and Trelawney [ 27 - 43 ]. These rural districts are situated in areas where communities survive on subsistence farming, growing maize, groundnuts, sunflowers and soya beans. While a few of the communities survive on market gardening and small-scale irrigation activities. Data from 1,008 adult participants enrolled in the 11 intervention communities of biomedical studies were included in this analysis. The observations in school children involved about 1,450 school children (95%CI: 120-173 per school) in 10 rural schools. We excluded individuals who were not willing to respond to the questionnaire at the baseline data collection points. Responding to the questionnaire at baseline indicated willingness to participate even though such individuals may not have participated at other subsequent follow-up time points.
Data collection
Data was collected in various methods including a survey. The survey of parents/guardians of school children participating in various immunological cohort and parasitlogy survey studies. Community advisory boards (CABs) members at 9 sites in the different study communities were also consulted and lastly 45 participants that including 40 Zimbabwean researchers and 5 international research collaborators. Community based field health workers who were part of the community and were also involved in study promotion and implementation activities collected data regarding participation. The field staffs were trained in interviewing and observation techniques, data recording, and participatory community motivation approaches. The field staff recorded willing participation indicators during the days of the follow-up with a structured, observational questionnaire. In addition, field staff recorded self-reported attendance at the end of the study follow-up point through a questionnaire. These were compared to the data recorded by the research teams at the field site and in the laboratory shown by the actual presence of the collected biological sample. In order to arrive at an outcome that describes willingness to participation, we selected six complementary survey indicators that measure multiple dimensions of potential willingness (Table 1 ). Based on this group separation, we used characteristics of participation in the groups to describe them in meaningful, qualitative terms: Group 1 = 'willing participants', Group 2 = 'moderate participants', Group 3 = 'availability of samples as willing to participate' Group 4 encouraged participants, Group 5 = 'organized participants' and Group 6 'participation due to self/community benefits.'
Indicators for study participation as classified according to groups and the rational or interpretation for analyses.
Statistical analysis
To identify patterns of study participation, we explored the quantitative distribution of study participation in terms of the six quantitative indicators (Table 1 ). Six differentiated groups were identified. To confirm the patterns of study participation we further examined the distribution of the willingness from samples obtained at examination day and the availability of samples, including reports from encouragement by the CABs as indicators. Willingness to participate measures in diverse communities and individual level characteristics were deduced between groups with data compared. The identified participation groups were then used in the comparison analyses. Data were analyzed using Statistical Package for Social Scientists (SPSS) version 8.0 for Windows, SPSS Inc. Chicago, USA.
Ethical approval
The biomedical studies obtained ethical approval from the Medical Research Council of Zimbabwe and also for each area, local leaderships were consulted and permission granted [ 27 - 43 ]. The provincial medical and education directorates provided permission while at the community level; the community leaders and CABs were first consulted for the main biomedical studies and also accepted the study. Informed consent was obtained from community leaders, adult participants, parents or guardians of school children prior to implementation of the main biomedical projects.
The field-based monitoring staff assessed the biomedical research compliance during baseline examination and at each subsequent follow-up time point in different studies conducted over a period of 6 years, from January 2005 to December 2010. The median duration of studies was 2 years, range: 0.5 year - 4 years, in 11 community-based studies, giving a total of 26 time points. At the community level, on average there were 95 participants per site (95% CI: 73 - 117), with participation patterns observed and a questionnaire administered. A total of 9 community advisory boards were interviewed (median 8 members/community, 95% CI: 7-12). Five of the studies involved taking blood samples while the rest involved epidemiological parasitology surveys and treatment programmes.
The level of participation varied, depending on the indicator used and the source of information. The encouragement by community-based staff (CABs) led to a moderate increase of 35% from the original 25% in the different communities. Participation compliance as observed by the research staff registered an increase during the follow-up visits where treatment was provided or a token of appreciation was given, with a median proportion of 80% (IQR: 65-95) in communities assessed. After 6 years of intensive research study implementation, the questionnaire administered by the research staff assessing study participation recorded 80% of respondents reported needing personal benefit from participation, and over 80% explicitly hinting on the need for revisions in determining the benefits for study participation. During the last intensive follow-up in each community the study staff deduced a declining willingness to participate, regardless of such benefits like provision of treatment for the examined parasite (Figure 1 ). By the end of the study, it was revealed that compensation and incentives were constantly being indicated as the main stimulation factor for adults' participation.
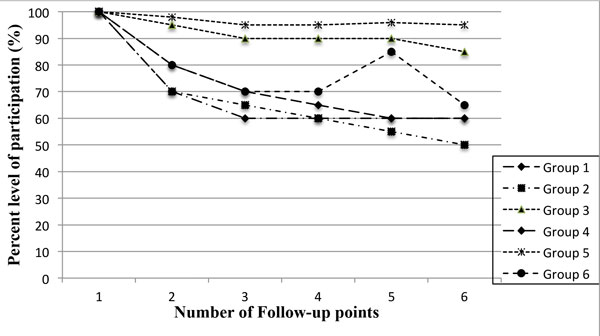
Showing the trends in levels of participation according to groups used in the analysis at different follow-up time points for the study sites summarized . The time points for each group may differ in year and type of study but the measurement was similar of the research subject willingness to participate.
Table 2 summarizes the results of the analysis, which identified six distinct participant groups based on purely on willingness to participate, and provision of all required samples as indicators. Group 5 (10 schools assessed), differed from the other groups with respect to the participants under observation as a type of a highly organized community with certain rules and regulation. While indicators 1 and 4 were more of community leaders and research staff noting upsurge of participation from records of samples obtained. These observations were noticed to decline with time into the study even though this recorded a high variability in all of the indicators as it was difficulty to base participation on the persuasion by the CABs members. Groups 2, 3 and 6 comprised indicators with the highest visible and tangible outcomes, while Group 2 with an initially high observation that declined over time, and group 4 showed a clear pattern when incentives were made available. Group 6 showed that regardless of the most important benefit to the participants of treatment available at no cost, participation was seen to decline drastically with time into the projected timeframe, an indication of probable participation fatigue. Table 2 , shows the difference between groups in 6 different participation indicators (research staff-reported, community observed) and two other monitoring indicators.
Level of attendance or provision of samples (%) at study sites for school children and adults relative to receiving incentives, compensation, re-imbursement and treatment
Table 2 , summarizes the need for compensation, incentives or re-imbursement through active participation at community level, through passive discussions with researchers and regulatory agents and at different institutions. Since the assessment/observation was standardized at community levels there is no difference between the six groups regarding features such as 'Number of follow-up events per community', 'Average number of participants per event and community', and 'Number of participation during the baseline and subsequently at each follow-up time point'. However, groups differed significantly regarding active participation at the events. School children in organized settings emerging to show high participation at all times at above 80% and 70% participation for parasitology/blood sampling and to receive treatment, respectively. The level of participation at school events was similar across groups, since participation was mandatory for school children in all schools in the study site (Table 2 ).
Participation group indicators correlated with each other and the estimates indicate that 'Total number of compliance by at least one indicator group' was positively associated with availability of samples of each group (Table 1 ). The results showed that availability of personal incentive, compensation or re-imbursement were more likely to enhance participation (OR: 3.38; 95% CI: 1.07-7.70) and to access any form of token given by researchers in community (OR: 2.02; 95% CI: 1.44-3.82). Furthermore, even school children from religious sector that do not take treatment was positively associated with increased participation when there is an incentive or a small token (provision of sweets or school writing notebooks) of appreciation (OR: 2.18; 95% CI: 1.17-3.20); an increase from 70% to 80% to receive treatment and a further increase from 35% to 80% encouraged to know their infection status when an incentives was introduced (Table 2 ).
About 90% (907) of the study participants and guardians expected a reward of reasonable value, in view of the researchers' value and in comparison to what is offered at other sites regardless of economic status of the community. Discussion among researchers at a basic ethics training workshop indicated that 80% (32) believed the decisions on level of incentive should be determined by the local research ethics committees (Table 3 ). While, the few international research collaborators were of the opinion that reward or compensation should be in accordance with local guidelines, and in line with funding as agreed and documented in the protocol during the design and reviews. The study revealed that participants and guardians were not happy about decision on level and type of incentive, reimbursement or compensation being reached on behalf of study participants without considering their expectations. On considering expectations of reward revealed that researchers should consult CAB members since they represent the community. In contrast to the adult participants and guardians of children involved in studies, who were of the opinion that compensation and incentives should be at individual level. Both the CAB members and study participants expressed that there should be a clear distinction between study incentive and compensation according to individual and community benefits from studies. Finally, CAB members expressed that the regulatory authorities that normally cite concerns unknown to them, often moderate their suggestions.
Preference of incentives or compensation given to study participants.
Realization of ethical principles by study participants in communities starts with researchers as they draw out the proposed protocol. These are upheld or authenticated by the ethics committees through reviewers as the study protocol goes through assessment and the approvals process. Further, data safety and monitoring boards (DSMBs) try to observe that ethical guidelines are maintained and there is no prejudice while testing the study hypotheses. While the regulatory authorities (e.g. Medicines Control Authorities, Medical Research Councils, etc.) verify that ethical principles are adhered to and practised during the conduct of the studies. However, governments through the ministry of health and other ministries overseeing research, sometimes take advantage of the research activities to fulfil their own political promises using resources supposed to be research incentives. Very often it is not rare to find some policy makers twisting the regulations to achieve certain goals for the communities. While rarely advocacy groups including NGOs represent the community leaders and the voiceless participants.
Lack of empowerment exposes African research participants and even African researchers and institutions to exploitation, coercion, enticement and inducement that would compromise overall voluntariness, and even upholding fairness is research studies. The sponsor and the investigator must take every effort to ensure that the research is responsive to the health needs and priorities of the population or community in which it is to be carried out. If the capacity is lacking, steps must be taken to strengthen the oversight mechanisms [ 4 ]. Usually, some key players are easily identified with major responsibilities in research, however, the concern in determining the respect for the research participant is often over looked. Most guidelines refer to research participants as mere study components to be protected disregarding the need for rewards and individual benefits. The hierarchy in research authority and all responsible overseers should understand the demands and needs of the communities they protect. Research in resource limited areas need to have prescriptive guidelines that accounts for individual desires for rewards. The research participant is a living individual from whom a researcher obtains data and specimens. The investigation is performed on the research participant that means the individual is central to the activities. Researchers and all responsible authorities have ethical and legal obligations to protect and satisfy human participants universally [ 3 , 13 ].
The fundamental ethical principle of justice requires fairness or entitlement that is giving to each what is due. Human beings are morally equal and should be treated as such regardless of colour, creed, race or religion including economic status. This principle of justice demands fairness in treatment of individuals and communities as such there should be equitable distribution of the burden and benefits of research. Important implications for such issues include rewards to participants during the study and post-study benefits. Generally, the communities in resource-constrained areas still do not enjoy the fruits of study participation at an individual level. The fundamental principle of autonomy requires that the wishes and choices of an individual be respected [ 2 ]. Individuals in research studies must be their own masters and can act or make free choices and take decisions without constraint of another. This is rare where no individual opportunity is given to make an informed decision to ask if not demand for reward, rather level and the regulatory authorities presume the type of reward, and very often the principle of divide and rule is applied. No discussion is permitted even though consent is obtained. Most resource-constrained communities do not exercise their demand rights but are entangled in mob participation that is taken advantage of by the area regulatory authorities under the pretext of representing the participants.
Most communities involved in studies or clinical trials are presumed to understand the essence of research. Even with these assumptions, participants should be given adequate information and explanation hence this implies that they volunteer to be objects of some experiments. Even though being aware that it is not an obligation to participate in the research, most resource constrained communities and individuals flow together without demanding or exercising the right of being free to refuse participation. The success of research is highly dependent on the willingness and cooperation of the participants during the protocol activities, by providing information or specimens. Informed consent plays an important part in this regard [ 1 , 2 , 7 ]. The responsibilities often are weighted on the investigator even though there are considerable responsibilities on the research participant. In most instances, community representatives are a major player in decision making in as far as research participation. Researchers and trial sponsors need to consult communities through transparent and meaningful participatory process that involve participants during the early stages in the design, development, implementation and monitoring of the study activities [ 18 ]. Consultations maybe arranged through local community leaders such as headmen, chiefs, community health workers and local civic leaders. Most communities where research activities have been conducted, certain mechanisms have been established for community engagement by establishing community advisory boards.
Researchers or investigators have mammoth responsibilities that include extreme caution on the vulnerable populations. The Helsinki Declaration mentions the observation of the benefits to the community [ 1 ]. The Investigator must make evaluation on the benefits to the communities and or individual. The sponsor or investigator should make every effort to ensure the work is responsive to the health needs and the priorities of the population or community in which the study is being conducted. After the study or intervention, the knowledge generated should be made available for the benefit of the population or community [ 1 - 4 , 7 ].
One challenge of assessing the effectiveness of biomedical field research implementation is the lack of a reliable, unbiased and accepted indicator to measure participation. Compliance with the biomedical research programme and intervention (e.g. epidemiology project, clinical trials or testing clinical tools) is an important indicator of a successful implementation strategy. To our knowledge, none of the several studies that measured study participation in relation to compensation and giving out incentives as effectiveness to improve participation assessed determinants of compliance directly. To date, the most common end-points used to assess compliance rely on statistics deducted from successful follow-up as the indirect observation of willingness to participate and these indicators are often assessed once, usually at the end of the intervention, and the reliability of these indicators is unknown. Self-reported compliance in the context of an interview is known to produce inflated results due to reporting bias. In this study we use six measures of direct observation and researcher tallying attendance from sample availability to create a score to classify participation according to 'willingness to participate' by being present at examination and interview day, and 'provision of samples' as required on appropriate days. However, this approach to participation and availability of sample classification uses components that can readily indicate magnitude of willingness. Agreeing to be part of the study forces the investigator to subjectively determine the acceptance of the study by the community. There is a need for objective methods to classify participants into distinct willing groups and also to consider views of the CABs and other community leaders.
In this article we present a detailed analysis of research study compliance among participants from resource limited settings who participated in different community based studies in rural Zimbabwe. The assessment detected a highly statistically significant demand for incentive in school children and in adults with an overall compliance of above 80% based on both community- health worker assessment and the research staff. Here, we use research data collected over a number of studies whose participant compliance was monitored by CABs and the study researchers to objectively deduce participation. We then use the classified groups to describe the participation determinants that are associated with the general researcher-participant attitude in resource-constrained setting.
Systematic reviews of biomedical studies and clinical interventions in developing countries reveal that majority of the global inhabitants have somehow been reached by certain forms of research programme that demand their participation. Further, from empirical research data, the world is now becoming a village in as far as being accessed by study programs is concerned. The global research events indicate that it is becoming increasingly difficulty to isolate communities from what was practiced in other areas during conduct of research programs. Even justification for grossly different levels of incentives, compensation or reimbursement can no longer continue, as the world becomes a global village. There is need for collective consideration of both personal and community rewards in future studies to be conducted in resource-constrained communities. Information available indicates a projected fear that recruitment in future may be a challenge, now that almost every community has somehow been reached and participated in some form of research studies. A major concern is that study participation rewards should be internationally pegged regardless of different economic status of the individuals or communities.
The challenge for incentives, compensation or even reimbursement is addressed unequally between regions and development status of the community or country. Resource constrained communities welcome many forms of studies regardless of the exploitation levels, due to poverty. Most of these communities are not empowered to air out their demands. Further, in such communities there is no recourse to challenge irrational health research policies and administrative decisions. If research intellectual and legal rights can be shared equitably between researchers and their institutions - what is preventing individualized benefits to study participants? We characterized six distinct participation groups in studies conducted over 6 years among participants of school- and community-based studies in rural Zimbabwe. Participation characteristics that were most strongly associated with the categorized groups include giving incentives, the level of compensation or reward for time taken to participation. These three forms of study participant benefit were strongly associated with participation. Promotion of efforts to give the study participants something would more easily encourage participation, and presumably reduce recruitment time.
Our findings suggest that the motivation to provide samples and to participate; even for treatment requires some form of rewards. In addition, higher compliance and obtaining samples was associated with the frequency of issuing out some token of appreciation to promote individual attendance at sample collection time point. It is likely that eager participants providing the biological samples are more interested in participating at the related promotional events and with incentives. Applying the theory and belief of due influence if incentives are used, has no place in designing studies in the modern research programme. These coherent findings on the motivating factors for participation underscore the importance of determining form and level of incentive for the participants prior to implementing the project. In combining objective indicators that measured visible signs of willing participation (e.g. provision of biological samples or being present to give the samples especially blood samples for immunological work) with proxies indicative of responsive to CABs encouragement and the presence of the biological samples collected at the required time point increased the quality of measurement and reduced the potential for reporting bias. The CABs evaluation on compliance generated much lower willingness rates than research staff on actual sample availability observation. This underscores the potential for bias in situations where community based staff as members of the CABs evaluate their own work through compliance after mobilization. Our results highlight the importance of choosing independent staff and a valid and responsive indicator to assess willingness and compliance and to draw conclusions about the need or effectiveness of incentives in intervention programme.
Despite an intensive baseline and continuous mobilization campaign carried out by research teams and CABs members, we observed 35% overall compliance without any reward given out at subsequent follow-up time point. However, when incorporating a simple token or reward in the form of dried fish and cooking oil, participation increased to over 70%. While during the follow-up when the rewards were not available, there was a reasonable response to participation on the first day but when participant realized that nothing was being given out, the attendance dropped drastically. Introduction of a small token or reward during follow-up showed an increase to treatment uptake, even when giving out treatment with sweetened orange crush juice to a sector of school children from a religious group that does not accept treatment. Our findings suggest that biomedical research programme would benefit from reassessing the core requirement for compliance. According to information from resourced communities, there are stark differences in marketing messages and approaches to reach the critical fraction of the population to participate in such studies. Our analysis identified some characteristics associated with increased willingness to participate, after receiving a small token or reward, indicating the potential to draw community members to the study. Most of the concerns can be assessed and addressed during the writing up of the protocols and incorporated during marketing and promotion strategies targeting the participants and informing on the personal benefits and rewards from participation. Based on the characteristics that we measured, it was clear to differentiate the willing participant from 'incentive driven participation (Table 2 ). In the study, the population of the participant groups included the most marginalized rural communities by observable characteristics: they were poor, lived further from health services centres, rarely had enough daily resources, the communities have high prevalence of neglected tropical diseases. We give evidence of the need to include individual token of appreciation. But the agents involved in designing and reviewing the protocols rarely would agree to reward participants in whatever form that the communities would appreciate.
In the resource constrained areas context, programme planning may benefit from assessing easy measurable factors like the individual desires and community expectations, a large proportion of population subgroups in these poor communities that can be targeted for biomedical research sites do not have excessive demands for compensation or rewards at individual level. Those insights supported by our data are consistent with recommendations for a successful rollout of clinical trials programme deriving from other previous studies. Central government suggested levels of appreciation for the communities are not in line with what the community and individuals expect and sometimes rarely would the communities receive these contributions from the researchers. Normally, it is not uncommon that resources from research programme get diverted or replaces government responsibilities, hence individual senior government officials mat benefit from such confusion. Individual benefits should be considered separate from government responsibilities where these are required. As a result, regulatory authorities very often propose making use of community rewards from study programme for community developments, thereby portraying differences to studies conducted in resourceful areas. The difference in not permitting the rewards to an individual is beyond any reasonable thinking besides among those who wish to deny these communities their dues from participation in studies.
There are limitations to this study. The participating communities were not homogenous regarding preexisting infrastructures, previous exposure to research campaigns and biomedical programme, as well as political support to participate in the study. Finally, data on the willing to participate and comparable time points where a reward was introduced, may somehow differ because (i) the indicator was implemented by different groups, and (ii) availability of samples and presence to uptake treatment in non-invasive biomedical studies. We believe such measurements somehow enhanced the reliability of willingness to participate due to a direct visible benefit and none invasiveness of the study procedure.
Conclusions
Analyses of implementation effectiveness and the willingness to participate in research programme are rarely published. Our findings suggest that individuals from resource-constrained settings are marginalized in deciding their fate and desires for their participation in research studies. This finding suggests how researchers could identify from the communities where studies are to be conducted, the general wish for rewards of the populations most likely to encourage participation in studies. Introduction of such rewards would be greatly beneficial to clinical, epidemiological and other similar studies by reducing recruitment time frame in reaching desired sample sizes. The key finding here is that communities feel marginalized in decision making. There is no clear conclusion on the view of the stakeholders on participation incentives, compensation and re-imbursement. The subjects need awareness on their rights under the principles of Universal declarations of Bioethics and Human Rights and other international normative instruments on life sciences research ethics.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
TM, NM, DD and PN conceived the idea and developed the design for the study. TM wrote the original draft manuscript, and incorporated revisions from each of the co-authors. NM and DD contributed to the conception and design of the manuscript and conducted the statistical analysis. TM and NM coordinated and supervised data acquisition. All authors read and approved the final manuscript.

Acknowledgements
We acknowledge the communities and the authorities of the Burma Valley District, Charehwa in Mutoko, Murehwa District, Goromonzi District, Kariba, Hurungwe, Shamva and Trelawney Districts. We acknowledge the collaboration with National Institute of Health Research, Ministry of Health & Child Welfare, Zimbabwe in the different research programme cited in this publication as part of their routine research programme on diseases of public health concern.
Funding for the study was part of the awards from Ministry of Health & Child Welfare, University of Zimbabwe Research Board funds, IFS and The Wellcome Trust. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript.
Declarations
Publication of this supplement has been funded by the College of Health Sciences and the Research Office at the University of Kwazulu-Natal.
This article has been published as part of BMC Medical Ethics Volume 14 Supplement 1, 2013: Selected papers from the 3rd Ethics, Human Rights and Medical Law Conference (3rd EHMRL). The full contents of the supplement are available online at http://www.biomedcentral.com/bmcmedethics/supplements/14/S1 .
- World Medical Association. Ethical principles for medical research involving human subjects. 2008. http://www.wma.net Seoul Korea: Declaration of Helsinki 59th WMA General assembly.
- Belmont Report. Ethical principles and guidelines for the protection of human subjects of research. http://www.hhs.gov/ohrp/humansubjects/guidance/belmont.html Report of the National commission for protection of the human subjects of biomedical and behavioral research; 1979.
- Nuffield Council on Bioethics. The ethics of research related to healthcare in developing countries. 2002. Nuffield council.
- International ethical guidelines for biomedical research involving human subjects. 2002. http://www.cioms.ch/publications Geneva: Council for international organization of medical sciences (CIOMS) in collaboration with World Medical Organization (WHO) [ PubMed ]
- Mduluza T. In: A Gateway to Biomedical Research in Africa. Mduluza T, Chima SC and Nsimba S, editor. New York: Nova Science Publishers; 2007. Towards community engagement; pp. 147–172. [ Google Scholar ]
- Ndebele P, Mfutso-Bengo J, Mduluza T. Compensating clinical trial participants from limited resource settings in internationally sponsored clinical trials: a proposal. Malawi Med J. 2008;14(2):42–5. doi: 10.4314/mmj.v20i2.10955. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Operational guidelines for ethics committees that review biomedical research. Geneva: World Health Organization (WHO); 2000. [ Google Scholar ]
- Hughes JR. Exclusion of "Noncompliant" individuals from clinical trials. Controlled Clinical Trials. 1993;14:176–7. doi: 10.1016/0197-2456(93)90019-A. [ DOI ] [ PubMed ] [ Google Scholar ]
- The Belmont Report | HHS.gov. 2011. http://www.hhs.gov/ohrp/humansubjects/guidance/belmont.html
- Council of Europe - ETS no. 164 - Convention for the Protection of Human study participants" 2003.
- Nuffield Council on Bioethics. The ethics of research related to healthcare in developing Countries. Latimer Trend Group. UK; 2002. [ Google Scholar ]
- Council of International Organizations of Medical Sciences. International Ethical Guidelines for Biomedical Research involving human subjects. 1993.
- WHO technical report. Guidelines for good clinical practice (GCP) for trials on pharmaceutical products. World Health Organization technical report series No. 850, 1995, annex 3.
- Nyika A, Kilama W, Chilengi R, Tangwa G, Tindana P. Capacity building of ethics reviews committees across Africa based on the results of a comprehensive survey. Developing World Bioethics. 2008;14:8731. doi: 10.1111/j.1471-8847.2008.00243.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Mudur G. John Hopkins admits scientist used Indian patients as guinea pigs. BMJ. 2001;14:7323. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dyer O. GP suspended for enrolling patients in drug trials without consent. BMJ. 2003;14:304. doi: 10.1136/bmj.326.7384.304. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Beauchamp LT, Childress FJ. Principles of Biomedical Ethics. 5. Oxford: Oxford University Press; 2001. [ Google Scholar ]
- Molyneux CS, Peshu N, Marsh K. Trust and Informed consent: Insights from community members on Kenyan Coast. Soc Sci Med. 2002;14:1463–1473. doi: 10.1016/j.socscimed.2004.11.073. [ DOI ] [ PubMed ] [ Google Scholar ]
- Nyika A, Kilama W, Chilengi R, Tangwa G, Tindana P, Ndebele P, Ikingura J. Composition, raining needs and independence of ethics review committees across Africa: are the gatekeepers rising to the emerging challenge? J Med Ethics. 2009;14:189–193. doi: 10.1136/jme.2008.025189. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Weijer C, Miller PB. Protecting communities in Pharmacogenetics and Pharmacogenomics research. Pharmacogenomics J. 2004;14:9–16. doi: 10.1038/sj.tpj.6500219. [ DOI ] [ PubMed ] [ Google Scholar ]
- World Health Organization. Geneva; 2004. World Health Organization. The Global Burden of Disease. [ Google Scholar ]
- Doyal L. Gender and 10/90 Gap in Health Research. Bull. World Health Organization. 2008;14 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Singh JA. Using the courts to challenge irrational health research [olicies and administration decisions. Acta Tropica. 2009;14:553–562. doi: 10.1016/j.actatropica.2009.07.035. [ DOI ] [ PubMed ] [ Google Scholar ]
- Pinheiro CP. Drug donations: what lies beneath. Bull World Health Organization. 2008;14(8):580–584. doi: 10.2471/BLT.07.048546. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lavery VJ, Grady C, Wahl RE, Emmanuel JE. Ethical issues in International Biomedical Research. A case Book. Oxford: Oxford University Press; 2007. [ Google Scholar ]
- Frost LJ, Reich MR. Harvard Centre for population and Development Studies. Cambridge, Massachussettes; 2008. Access: How Do Health technologies get to the poor people in the poor countries? [ Google Scholar ]
- Appleby LA, Nausch N, Midzi N, Mduluza T, Allen JE, Mutapi F. Sources of heterogeneity in human monocyte subsets. Immunol Letters. 2013;14:32–41. doi: 10.1016/j.imlet.2013.03.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mduluza T, Gori E, Chitongo P, Midzi N, Ruwona T, Soko W, Mlambo G, Mutambu SL, Kumar N. Analysis of TNF-α and IL-10 gene polymorphisms in Zimbabwean children exposed to malaria. African J of Biotech. 2013. pp. 1034–1039.
- Nausch N, Louis D, Lantz O, Peguillet I, Trottein F, Chen IY, Appleby LJ, Bourke CD, Midzi N, Mduluza T, Mutapi F. Age-Related Patterns in Human Myeloid Dendritic Cell Populations in People Exposed to Schistosoma haematobium Infection. PLoS Negl Trop Dis. 2012;14(9):e1824. doi: 10.1371/journal.pntd.0001824. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Reilly L, Nausch N, Midzi N, Mduluza T, Mutapi F. Association between Micronutrients (Vitamin A, D, Iron) and Schistosome-Specific Cytokine Responses in Zimbabweans Exposed to Schistosoma haematobium. J Parasitol Res. 2012. p. 128628. [ DOI ] [ PMC free article ] [ PubMed ]
- Rujeni N, Nausch N, Bourke CD, Midzi N, Mduluza T, Taylor DW, Mutapi F. Atopy Is Inversely Related to Schistosome Infection Intensity: A Comparative Study in Zimbabwean Villages with Distinct Levels of Schistosoma haematobium Infection. Int Arch Allergy Immunol. 2012;14:288–298. doi: 10.1159/000332949. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Midzi N, Mtapuri-Zinyowera S, Sangweme D, Paul NH, Makware G, Mapingure MP, Brouwer KC, Mudzori J, Hlerema G, Chadukura V, Mutapi F, Kumar N, Mduluza T. Efficacy of integrated school based de-worming and prompt malaria treatment on helminths -Plasmodium falciparum co-infections: A 33 months follow up study. BMC Int Health Hum Rights 2011. pp. 9–18. [ DOI ] [ PMC free article ] [ PubMed ]
- Midzi N, Mtapuri-Zinyowera S, Mapingure MP, Paul NH, Sangweme D, Hlerema G, Mutsaka MJ, Tongogara F, Makware G, Chadukura V, Brouwer KC, Mutapi F, Kumar N, Mduluza T. Knowledge attitudes and practices of grade three primary schoolchildren in relation to schistosomiasis, soil transmitted helminthiasis and malaria in Zimbabwe. BMC Infect Dis. 2011;14:11–169. doi: 10.1186/1471-2334-11-169. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mutapi F, Rujeni N, Bourke C, Mitchell K, Appleby L, Nausch N, Midzi N, Mduluza T. Schistosoma haematobium treatment in 1-5 year old children: safety and efficacy of the antihelminthic drug praziquantel. PLoS Negl Trop Dis. 2011;14(5):e1143. doi: 10.1371/journal.pntd.0001143. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mduluza T, Mutapi F, Ruwona T, Kaluka D, Midzi N, Ndhlovu PD. Similar cellular responses after treatment with either praziquantel or oxamniquine in Schistosoma mansoni infection. Malawi Med J. 2009;14(4):176–82. doi: 10.4314/mmj.v21i4.49642. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sangweme DT, Midzi N, Zinyowera-Mutapuri S, Mduluza T, Diener-West M, Kumar N. Impact of schistosome infection on Plasmodium falciparum Malariometric indices and immune correlates in school age children in Burma Valley, Zimbabwe. PLoS Negl Trop Dis. 2010;14(11):e882. doi: 10.1371/journal.pntd.0000882. 9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Midzi N, Mtapuri-Zinyowera S, Mapingure MP, Sangweme D, Chirehwa MT, Brouwer KC, Mudzori J, Hlerema G, Mutapi F, Kumar N, Mduluza T. Consequences of polyparasitism on anaemia among primary school children in Zimbabwe. Acta Trop. 2010;14(1-2):103–11. doi: 10.1016/j.actatropica.2010.02.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kjetland EF, Hove RJ, Gomo E, Midzi N, Gwanzura L, Mason P, Friis H, Verweij JJ, Gundersen SG, Ndhlovu PD, Mduluza T, Van Lieshout L. Schistosomiasis PCR in vaginal lavage as an indicator of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg. 2009;14(6):1050–5. doi: 10.4269/ajtmh.2009.09-0081. [ DOI ] [ PubMed ] [ Google Scholar ]
- Mtapuri-Zinyowera S, Midzi N, Muchaneta-Kubara EC, Simbini T, Mduluza T. Impact of solar radiation in disinfecting drinking water contaminated with Giardia duodenalis and Entamoeba histolytica ⁄dispar at a point-of-use water treatment. J Appl Microbiol. 2009. pp. 847–852. [ DOI ] [ PubMed ]
- Midzi N, Butterworth AE, Mduluza T, Munyati S, Deelder AM, van Dam GJ. Use of circulating cathodic antigen strips for the diagnosis of urinary schistosomiasis. Trans R Soc Trop Med Hyg. 2009;14(1):45–51. doi: 10.1016/j.trstmh.2008.08.018. [ DOI ] [ PubMed ] [ Google Scholar ]
- Mlambo G, Mutambu SL, Mduluza T, Soko W, Mbedzi J, Chivenga J, Lanar DE, Singh S, Carucci D, Gemperli A, Kumar N. Antibody responses to Plasmodium falciparum vaccine candidate antigens in three areas distinct with respect to altitude. Acta Trop. 2006;14(1-2):70–8. doi: 10.1016/j.actatropica.2006.09.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- Ndhlovu PD, Mduluza T, Kjetland EF, Midzi N, Nyanga L, Gundersen SG, Friis H, Gomo E. Prevalence of urinary schistosomiasis and HIV in females living in a rural community of Zimbabwe: does age matter? Trans R Soc Trop Med Hyg. 2007;14(5):433–8. doi: 10.1016/j.trstmh.2006.08.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kjetland EF, Ndhlovu PD, Gomo E, Mduluza T, Midzi N, Gwanzura L, Mason PR, Sandvik L, Friis H, Gundersen SG. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006;14(4):593–600. doi: 10.1097/01.aids.0000210614.45212.0a. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (369.0 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Medical Oncologists' Perceptions of Financial Incentives in Cancer Care
Jennifer l malin, jane c weeks, arnold l potosky, mark c hornbrook, nancy l keating.
- Author information
- Article notes
- Copyright and License information
Corresponding author: Jennifer L. Malin, MD, PhD, WellPoint, 1 WellPoint Way, Thousand Oaks, CA 91362; e-mail: [email protected] .
Issue date 2013 Feb 10.
The cost of cancer care continues to increase at an unprecedented rate. Concerns have been raised about financial incentives associated with the chemotherapy concession in oncology practices and their impact on treatment recommendations.
The objective of this study was to measure the physician-reported effects of prescribing chemotherapy or growth factors or making referrals to other cancer specialists, hospice, or hospital admissions on medical oncologists' income. US medical oncologists involved in the care of a population-based cohort of patients with lung or colorectal cancer from the Cancer Care Outcomes Research and Surveillance (CanCORS) study were surveyed regarding their perceptions of the impact of prescribing practices or referrals on their income.
Although most oncologists reported that their incomes would be unaffected, compared with salaried oncologists, physicians in fee-for-service practice, and those paid a salary with productivity incentives were more likely to report that their income would increase from administering chemotherapy (odds ratios [ORs], 7.05 and 7.52, respectively; both P < .001) or administering growth factors (ORs, 5.60 and 6.03, respectively; both P < .001).
A substantial proportion of oncologists who are not paid a fixed salary report that their incomes increase when they administer chemotherapy and growth factors. Further research is needed to understand the impact of these financial incentives on both the quality and cost of care.
INTRODUCTION
Since former President Nixon declared war on cancer four decades ago, the cost of treating cancer has increased dramatically as a result of new diagnostic and treatment technologies and pharmaceutical and biologic treatment innovations. The annual cost of cancer care in the United States exceeded $124 billion in 2010 and is projected to be $173 billion in 2020. 1
In addition to the rising cost of initial cancer treatment, 2 care is increasingly aggressive near the end of life, with greater use of chemotherapy just before death, more visits to the emergency department, more hospitalizations, and more admissions to the intensive care unit. 3 – 5
Most oncology services are reimbursed as independent units, with separate payments for physician services, diagnostic testing, and chemotherapy administration. 6 Thus, similar to most US physicians, medical oncologists' compensation is generally based on their productivity—that is, the more services they provide, the more they bill, and the greater their own individual compensation. 7 The practice of medical oncology involves the administration of chemotherapy and supportive medications, such as growth factors, which has led to many private practices having infusion centers in their offices. The chemotherapy concession is the source of approximately 65% of the revenue in a typical oncology practice dwarfing the income from evaluation and management. 8 The delivery of chemotherapy in physicians' offices has potential advantages for patients because they may be able to receive treatment in their own community and not have to travel to a hospital for their cancer therapy. However, oncologists who administer chemotherapy in their offices have traditionally charged payers more than their cost for acquiring the drugs, so there is potential for financial conflicts of interest to influence their prescribing behavior. 6 , 9 – 12 Indeed, it has been suggested that the increased income to physicians from prescribing growth factors has contributed to their widespread adoption. 13 Despite these anecdotes from the press, little is known about the actual impact of prescribing chemotherapy or growth factors on medical oncologists' income.
We used survey data from medical oncologists participating in the Cancer Care Outcomes Research and Surveillance (CanCORS) study 14 to compare the perceived effects on personal income of administering chemotherapy and growth factors, referring patients to other cancer specialists, referring patients to hospice, and admitting patients to the hospital. In addition, we sought to understand how physician and practice characteristics influence physicians' perception that ordering any of these services would increase their income.
Study Design
Data for this study were collected as part of a national study of variations in care and outcomes of care for patients with lung or colorectal cancer undertaken by the CanCORS Consortium. 10 CanCORS examined care delivered to population-based cohorts totaling more than 10,000 patients initially diagnosed with lung or colorectal cancer in 2003 to 2005 living in Northern California, Los Angeles County, North Carolina, Iowa, or Alabama or who received care in one of five large health maintenance organizations (HMOs) or 15 Veterans Health Administration (VHA) study sites. Data were collected from patient interviews, medical record abstractions, physician surveys, and surveys of informal caregivers. The study was approved by the human subjects committees at all participating institutions; the investigators obtained informed consent from each participant or each participant's guardian. This analysis uses data only from physician surveys.
Study Population
We surveyed medical oncologists, surgeons, radiation oncologists, and primary care providers named by the patients participating in the CanCORS study as serving important roles in their cancer care from January 2005 through March 2007. Details regarding the physician survey methodology, including characterization of nonresponders, have been published previously. 15 This study includes only the medical oncologists who participated in the survey. Of 908 physicians who provided (or discussed) chemotherapy for CanCORS participants for whom contact information was verified, 556 (61%) responded. We included physicians who reported medical oncology as their specialty (n = 495) and excluded respondents who did not respond to the items regarding the outcomes of interest (n = 15), resulting in a final study sample of 480.
Survey Instrument
To understand the physician-reported effects on their personal income of ordering various services or referring patients for various services, medical oncologists were asked “How, if at all, is your income most likely to change as a result of (a) referring more patients to other cancer specialists; (b) enrolling more patients in hospice; (c) admitting more patients to the hospital; (d) enrolling more patients in clinical trials; (e) prescribing/administering more chemotherapy; and (f) prescribing/administering more growth factor (eg, granulocyte colony-stimulating factor).” Response choices included: (1) Likely to increase, (2) Likely to decrease, (3) Not likely to change, and (4) Don't know. Physicians also provided information about personal and practice characteristics, including practice type (characterized as HMO, VHA/government, solo office, single-specialty office group, multispecialty office group, hospital), proportion of patients enrolled in managed care plans, proportion of patients reimbursed on a capitated or prepaid basis, dependence of salary on productivity (asked only of salaried physicians), and among nonsalaried physicians, type of base clinical income (exclusively fee-for-service, predominantly fee-for-service, equal mixture of fee-for-service and capitation, predominantly capitation, and exclusively capitation). An analysis of factors influencing physicians' enrollment of patients in clinical trials has been published separately and is therefore not included here. 16
Statistical Analysis
Overall, nonresponse to items was less than 2% to 3% for most variables. We used multiple imputation to impute missing data for these items. 17 We dichotomized the response choices into income likely to increase and income not likely to increase, including in the latter category the responses “not likely to change,” “likely to decrease,” and “don't know.”
Since only 1% of physicians reported that their income would be likely to increase with referrals to other cancer specialists or hospice and just 11% reported that income was associated with hospitalizations, these outcomes were not considered in subsequent analyses. We used χ 2 tests to assess the association of physician and practice characteristics and the dichotomized response regarding the effect on income of prescribing/administering more chemotherapy and prescribing/administering more growth factors. Specifically, we assessed age, sex, race/ethnicity, board certification, United States or Canadian medical school graduate, teaching involvement, practice type, percentage of patients in managed care, study site, practice at a National Cancer Institute (NCI) –designated cancer center, and physician base payment (salary not based on productivity, salary based on productivity, predominantly fee-for-service, or mixture of fee-for-service and capitation [with at least 50% capitation]). We used logistic regression to estimate the independent associations of physician and practice characteristics with physicians' perceptions that their income would be increased by administering chemotherapy or growth factors.
Just over half the respondents (52%) were age 50 years or older, 23% were female, 78% were graduates of US or Canadian medical schools, 92% were board certified, and 51% were engaged in teaching. Other characteristics of the physicians are delineated in Table 1 .
Physician and Practice Characteristics Associated With Self-Report That Referrals or Prescribing Increases Income
NOTE. No. of physicians missing data on specific characteristics: two, age; one, US medical school graduate; two, board certification; six, teaching status; 12, base clinical payment; 12, NCI cancer center.
Abbreviations: HMO, health maintenance organization; NCI, National Cancer Institute; VHA, Veterans Health Administration.
Most medical oncologists reported that their income was unaffected by their prescribing of chemotherapy or growth factors or referrals to other cancer specialists, hospice, or hospital admissions ( Fig 1 ). However, 27% indicated that their income would be increased by administering more chemotherapy, 25% by prescribing more growth factors, and 11% by admitting more patients to the hospital.
Impact of referrals and prescribing on medical oncologist income.
Although several factors were associated with a greater likelihood of reporting a positive association between income and the two outcomes of interest ( Table 1 ), in multivariate analyses practice characteristics and physician payment models were most strongly associated with income ( Table 2 ). After controlling for payment model and type of practice, the amount of time spent teaching and practicing at an NCI cancer center were no longer associated with increased income for any of the outcomes; however, graduates of US or Canadian medical schools were significantly more likely to report that prescribing growth factors increased their income compared with graduates of foreign medical schools (odds ratio [OR], 2.99; 95% CI, 1.39 to 6.44).
Adjusted Odds of Physician Self-Report That Prescribing Growth Factors or Chemotherapy Increases Their Income (N = 480)
Abbreviations: HMO, health maintenance organization; NCI, National Cancer Institute; OR, odds ratio; VHA, Veterans Health Administration.
Compared with physicians who were paid a salary not based on productivity, the odds of oncologists reporting that their income would increase with administration of chemotherapy were 7.52 (95% CI, 3.29 to 17.23) for those with mostly fee-for-service payment, 7.05 (95% CI, 3.22 to 15.43) for those paid a salary based on productivity, and 7.03 (95% CI, 1.55 to 31.73) for those paid fee-for-service and capitation (with > 50% capitation). Physicians in solo and single-specialty group practice were significantly more likely to report increased income from ordering chemotherapy (OR, 9.90; 95% CI, 2.75 to 35.60 and OR, 7.18; 95% CI, 2.43 to 21.19, respectively) relative to oncologists in hospital-based practices, controlling for all other factors. Similarly, physicians paid on a fee-for-service basis or by salary based on productivity were more likely to report that their incomes increased from prescribing growth factors (OR, 5.60; 95% CI, 2.47 to 12.71 and OR, 6.03; 95% CI, 2.48 to 13.79, respectively). Compared with those in hospital-based practices, oncologists in solo and single-specialty practices were significantly more likely to report a positive association between their incomes and administration of growth factors (OR, 11.28; 95% CI, 2.08 to 61.30 and OR, 18.76; 95% CI, 4.00 to 87.93). Medical oncologists in HMOs or government health care facilities were not significantly more likely than hospital-based physicians to report a positive impact of administering chemotherapy or growth factors on personal income.
The results of this study highlight the financial incentives of physicians in the US health care system that may be contributing to rising cancer care costs. A substantial proportion of medical oncologists reported that their income would increase with administering more chemotherapy or growth factors. However, this finding varied tremendously across practice type and physician payment model. In all, 40% to 50% of physicians whose incomes were based on fee for service or who received a salary with productivity incentives indicated that their income increased when they prescribed chemotherapy or growth factors. Physicians in solo single-specialty practice were also much more likely to perceive income increases than physicians in other types of practice settings. These findings for payment model and practice type persisted, even after adjustment for other confounding physician and practice characteristics. The results of this study corroborate and extend the findings from a previous analysis we published 18 that used data on the actual prescribing of growth factors for patients treated by physicians in this cohort. It showed that 96% of growth factor use in patients with lung and colorectal cancer occurred in situations in which the chemotherapy regimen did not put them at high risk of febrile neutropenia according to the guidelines and thus represented discretionary use of the therapy. Enrollment in an HMO was strongly associated with a lower adjusted odds of discretionary growth factor use, compared with patients not enrolled in an HMO.
Interestingly, graduates of US and Canadian medical schools were significantly more likely to report financial incentives for prescribing growth factors than graduates of foreign medical schools, even after adjusting for other physician practice characteristics; however, this association was not present for administering chemotherapy. To the best of our knowledge, this difference in perceived financial incentives has not previously been described and the reasons for it are unclear. Some evidence suggests that international medical school graduates may be more likely than other physicians to care for underserved patients, practice in rural areas, and offer volunteer community health care services. 19 Differences in cultural values may result in foreign medical school graduates approaching their medical practice with less emphasis on financial success. Alternatively, graduates of international medicals schools may have a lower debt burden when they enter into practice, and this may have an impact on their perception of the financial exigencies of practice. 20 This finding underscores the need for further research to elucidate the complex relationship between financial incentives, penalties, and provider behavior.
Because the United States struggles with how best to control health care costs, our results have important implications. Although physician services represent only 20% of health care costs, by ordering health care services, physicians' decisions are responsible for up to 90% of health care expenditures. 21 , 22 With the predominance of the private practice model, most physicians are independent practitioners who bill third-party payers for their services. Although capitation and risk-sharing arrangements have been a heavily relied on approach to control health care costs, we found that few oncologists received a substantial portion of their compensation through capitation arrangements. Nevertheless, these physicians were also more likely than physicians paid by salary not based on productivity to report that their income increased from prescribing chemotherapy. Only salaried physicians without any productivity incentives appeared to be free of financial incentives to administer chemotherapy or growth factors. The fact that physicians who received more than half their income from capitated agreements still had financial incentives to prescribe chemotherapy and growth factors could indicate that pharmacy services are not usually included in risk-sharing arrangements, although it is important to note that only 11 physicians in our cohort reported substantial income from capitation. Capitation schemes that include cancer therapy have not been widely adopted because the high cost of cancer care makes risk-sharing too risky for small practices and because of concerns that such arrangements could lead to withholding of necessary care. 23 , 24 These findings have important implications for accountable care organizations (ACOs), which involve risk-sharing arrangements between hospitals and physicians, currently being advocated as a potential solution to control Medicare costs. 25 Our study suggests that ACOs may have little impact on incentives to increase use of cancer services unless the ACO assumes full risk for cancer services and oncologists are reimbursed only for their clinical services or paid a salary.
Beyond a major overhaul of the US health care system, which would be required if all oncologists were to be salaried, how can the financial inducements to administer chemotherapy and supportive care therapies be decreased? Specialty pharmacy programs that provide cancer drugs to the oncologist's office for infusion, thereby eliminating the chemotherapy concession, are one potentially effective strategy, since they remove any financial incentive associated with administering chemotherapy and growth factors. In a recent pilot project, a managed care organization decreased injectable drug costs by 15% by purchasing drugs through a specialty pharmacy program and supplying the drugs to be infused instead of allowing physicians' offices to “buy and bill.” 26 However, there are many challenges to successful implementation, including issues related to storage, administration, and concerns about waste if site-of-care laboratory testing indicates that the drug should not be given. 27 Another approach that has been proposed involves paying providers for episodes of care (eg, adjuvant therapy for stage III colon cancer) in which the payment would cover all costs of care, including chemotherapy and supportive care drugs. 28 The impact of this and other similar approaches on the cost and quality of cancer care is not yet known.
Our results should be viewed in light of several limitations. First, our findings are based on physician self-report of the impact of administering chemotherapy and growth factors on physician income. We do not know whether this accurately reflects the impact of these services on their income, whether and how often oncologists are influenced by this incentive, or the magnitude of this effect. In the most recent National Oncology Benchmark Report, 8 practices reported that, on average, 65% of their revenue was from administration of drugs, suggesting that these financial incentives can be substantial. One could argue that such incentives are not necessarily harmful and may in fact be of benefit if they result in more patients receiving high-quality, efficient care. However, as previously mentioned, 96% of growth factor use in the patients cared for by the same physicians included in our analysis was inappropriate according to the guideline recommendations at the time, suggesting that these financial incentives are contributing to waste in the health care system. 14 Finally, it should be noted that we did not explore factors that could lead to a decrease in physicians' income. Because the amount that most payers will reimburse for drugs has substantially decreased over the last decade, some oncologists may find that reimbursements for some drugs may not cover their cost. Although only a few respondents to our survey reported that their income would decrease as a result of administering chemotherapy or prescribing growth factors, this may become more of a concern as cost containment efforts continue to exert pressure on reimbursement.
In conclusion, a substantial proportion of oncologists who are not paid a fixed salary report that their income increases when they administer chemotherapy and growth factors. New payments models are needed to counter or eliminate these incentives to decrease unnecessary care and ensure that health care resources are used most effectively.
Acknowledgment
We thank Yang Xu, MS, for assistance with statistical programming.
See accompanying editorial on page 517
Supported by Grants No. U01 CA093344 from the National Cancer Institute (NCI) to the Statistical Coordinating Center, U01 CA093332 from the Dana-Farber Cancer Institute/Cancer Research Network, U01 CA093324 from Harvard Medical School/Northern California Cancer Center, U01 CA093348 from RAND/University of California, Los Angeles, U01 CA093329 from the University of Alabama at Birmingham, U01 CA01013 from the University of Iowa, U01 CA093326 from the University of North Carolina, and CRS 02-164 from the Department of Veterans Affairs (VA) to the Durham VA Medical Center.
The views and opinions of authors expressed herein do not necessarily state or reflect the Department of Veterans Affairs, the United States Government, Kaiser Permanente, or WellPoint.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Jennifer L. Malin, WellPoint (C) Consultant or Advisory Role: Jennifer L. Malin, Onyx Pharmaceuticals (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Jennifer L. Malin, Jane C. Weeks, Mark C. Hornbrook, Nancy L. Keating
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
- 1. Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Warren JL, Yabroff KR, Meekins A, et al. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100:888–897. doi: 10.1093/jnci/djn175. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22:315–321. doi: 10.1200/JCO.2004.08.136. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer near the end of life: Is it a quality-of-care issue? J Clin Oncol. 2008;26:3860–3866. doi: 10.1200/JCO.2007.15.8253. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Sharma G, Freeman J, Zhang D, et al. Trends in end-of life ICU use among older adults with advanced lung cancer. Chest. 2008;133:72–78. doi: 10.1378/chest.07-1007. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Bach PB. Costs of cancer care: A view from the Centers for Medicare and Medicaid Services. J Clin Oncol. 2007;25:187–190. doi: 10.1200/JCO.2006.08.6116. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Reschovsky JD, Hadley J. Physician Financial Incentives: Use of Quality Incentives Inches Up, but Productivity Still Dominates. Issue Brief No. 108, January 2007. http://hschange.org/CONTENT/905/#ib6 . [ PubMed ]
- 8. Barr TR, Towle EL. National oncology practice benchmark: An annual assessment of financial and operational parameters—2010 report on 2009 data. J Oncol Pract. 2011;7(suppl 2):2s–15s. doi: 10.1200/JOP.2011.000223. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. General Accounting Office. Washington, DC: General Accounting Office; 2001. Medicare: Payments for covered outpatient drugs exceed providers costs. GAO-01-1118. [ Google Scholar ]
- 10. Elliott SP, Jarosek SL, Wilt TJ, et al. Reduction in physician reimbursement and use of hormone therapy in prostate cancer. J Natl Cancer Inst. 2010;102:1826–1834. doi: 10.1093/jnci/djq417. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Jacobson M, Earle CC, Price M, et al. How Medicare's payment cuts for cancer chemotherapy drugs changed patterns of treatment. Health Aff (Millwood) 2010;29:1391–1399. doi: 10.1377/hlthaff.2009.0563. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Smith TJ, Hillner BE. Concrete options and ideas for increasing value in oncology care: The view from one trench. Oncologist. 2010;15:65–72. doi: 10.1634/theoncologist.2010-S1-65. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Berenson A, Pollack A. Doctors reap millions for anemia drugs. New York Times. 2007 May 9; http://www.nytimes.com/2007/05/09/business/09anemia.html?_r=1&scp=44&sq=&st=nyt . [ Google Scholar ]
- 14. Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: The Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22:2992–2996. doi: 10.1200/JCO.2004.06.020. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Keating NL, Landrum MB, Klabunde CN, et al. Adjuvant chemotherapy for stage III colon cancer: Do physicians agree about the importance of patient age and comorbidity? J Clin Oncol. 2008;26:2532–2537. doi: 10.1200/JCO.2007.15.9434. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Klabunde CN, Keating NL, Potosky AL, et al. A population-based assessment of specialty physician involvement in cancer clinical trials. J Natl Cancer Inst. 2011;103:384–397. doi: 10.1093/jnci/djq549. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. He Y, Zaslavsky AM, Landrum MB, et al. Multiple imputation in a large-scale complex survey: A practical guide. Stat Methods Med Res. 2010;19:653–670. doi: 10.1177/0962280208101273. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Potosky AL, Malin JL, Kim B, et al. Use of colony-stimulating factors with chemotherapy: Opportunities for cost savings and improved outcomes. J Natl Cancer Inst. 2011;103:979–982. doi: 10.1093/jnci/djr152. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Bazemore AW, Goldenhar LM, Lindsell CJ, et al. An international health track is associated with care for underserved US populations in subsequent clinical practice. J Grad Med Educ. 2011;3:130–137. doi: 10.4300/JGME-D-10-00066.1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Gozu A, Kern DE, Wright SM. Similarities and differences between international medical graduates and U.S. medical graduates at six Maryland community-based internal medicine residency training programs. Acad Med. 2009;84:385–390. doi: 10.1097/ACM.0b013e318197321b. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Kaiser Family Foundation. U.S. health care costs: Background brief. 2010. Mar, http://www.kaiseredu.org/topics_im.asp?imID=1&parentID=61&id=358 .
- 22. Eisenberg JM. Physician utilization: The state of research about physicians' practice patterns. Med Care. 2002;40:1016–1035. doi: 10.1097/01.MLR.0000032181.98320.8D. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Zarkowsky H. Oncology services for members of a national managed care company. Cancer. 1998;82:2039–2042. doi: 10.1002/(sici)1097-0142(19980515)82:10+<2039::aid-cncr11>3.0.co;2-c. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Butcher L. US Oncology prepares for risk-based contracts. Oncology Times. 2010;32:25–26. [ Google Scholar ]
- 25. Berwick DM. Launching accountable care organizations: The proposed rule for the Medicare Shared Savings Program. N Engl J Med. 2011;364:e32. doi: 10.1056/NEJMp1103602. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Baldini CG, Culley EJ. Estimated cost savings associated with the transfer of office-administered specialty pharmaceuticals to a specialty pharmacy provider in a Medical Injectable Drug program. J Manag Care Pharm. 2011;17:51–59. doi: 10.18553/jmcp.2011.17.1.51. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Pane FJ. White bagging: A new challenge for your hospital. Pharmacy Practice News. 2009;36:12. http://pharmacypracticenews.com/ViewArticle.aspx?d=Operations+&+Management&d_id=53&i=December+2009&i_id=587&a_id=14378 . [ Google Scholar ]
- 28. Bach PB, Mirkin JN, Luke JJ. Episode-based payment for cancer care: A proposed pilot for Medicare. Health Aff (Millwood) 2011;30:500–509. doi: 10.1377/hlthaff.2010.0752. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (107.2 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
' + document.title + '

- Popular Products Riskworkstation™ NCCI Atlas Class Look-Up Data Manager Dashboard

- Corporate Responsibility Facts and Information NCCI Cares Contact NCCI
- Learn About NCCI About Us Our Leadership Media Center NCCI State Map

- Other Learning Experiences NCCI Atlas Training Virtual Carrier Education Series Residual Market Training --> Microlearning Videos
- My Contact Information My Email Notifications Recent ncci.com Updates My Products Company Administrators
- Issues Tracking View/Pay My Invoice (OIS)

- Data Reporting
- Industry Information
- Residual Markets
- Underwriting
- Agents/Brokers

- My Contact Information
- My Email Notifications
- Recent ncci.com Updates
- My Products
- Company Administrators
- Issues Tracking
- View/Pay My Invoice (OIS)
- Product Catalog
- Riskworkstation™
- Class Look-Up
- Data Manager Dashboard
- Our Leadership
- Media Center
- NCCI State Map
- Corporate Responsibility
- Facts and Information
- Contact NCCI
- Explore Careers
- Learning Center
- NCCI Academy
- NCCI Atlas Training
- Virtual Carrier Education Series
- Residual Market Training
- Microlearning Videos

Mega claims, with reported losses above $2 million ($2M), represent less than 0.1% of total workers’ compensation (WC) claims but account for over 2% of total loss dollars, in excess of $1 billion each year.
These claims typically have significant medical expenses, stemming from severe injuries with prolonged recovery and time away from work. In response to stakeholder concerns about the relative frequency of these claims, several WC rating bureaus, the Workers’ Compensation Insurance Rating Bureau of California, Delaware Compensation Rating Bureau, Indiana Compensation Rating Bureau, Compensation Advisory Organization of Michigan, Minnesota Workers’ Compensation Insurers Association, National Council on Compensation Insurance, New Jersey Compensation Rating and Inspection Bureau, New York Compensation Insurance Rating Board, North Carolina Rate Bureau and Pennsylvania Compensation Rating Bureau produced a countrywide analysis of mega claims in 2020. These same bureaus have collaborated again to conduct an updated and enhanced analysis of the trends in frequency, characteristics, loss size, and emergence of countrywide mega claims.
Some drivers related to the cost of these mega claims include medical advances which improve patient outcomes and provide life-saving measures. Mega claim costs could be influenced by innovative rehabilitation technology, such as robotics and virtual reality, rising home health care costs, extended recovery times, and inflationary trends on services not explicitly included in a state’s medical fee schedule. Other innovations in the workplace, like safety technology and automation, may reduce the number of mega claims.
With four additional accident years, the research team revisits some questions from the previous study, such as whether mega claims are becoming more common or being recognized more quickly than in the past.
The study analyzes the emergence of mega claims, defined as claims with incurred loss at 2022 cost levels of at least $2M, unless a different threshold of $3M, $5M or $10M is specified. The updated analysis includes more granular industry group and claim characteristics for claims above $2M and calculates frequency relative to premium and ground-up claims. A section on severity is also included, analyzing patterns for mega claims above $2M.
Key Findings
- A total of 11,330 claims from accident years 2001 through 2021 were reported as of December 31, 2022, with incurred loss in excess of $2M at 2022 cost levels, which is approximately one out of every 1,295 reported indemnity claims. Of those, 53% were between $2M and $3M, 27% were between $3M and $5M, 15% were between $5M and $10M, and 4% were in excess of $10M.
- Since 2013, the estimated ultimate frequency of mega claims per 100,000 indemnity claims has been steadily increasing. This trend is less pronounced when counts of mega claims are compared to premium. Earlier recognition of mega claims has complicated the estimation of ultimate frequency as emergence of mega claims more than 18 months after policy inception has slowed down, consistent with the hypothesis of earlier recognition of mega claims.
- Frequency has increased across all industries with the largest increase in construction. Mega claims are being recognized earlier across industries.
- The share of claims greater than $5M is higher than those in the $2M to $5M range for injuries in Construction to the head and brain and from motor vehicles. Claims with these characteristics also represent a larger share of loss in excess of $2M.
- Claims in the highest severity categories are also the fastest to emerge. The categories with slowest emergence are office and clerical, lower back, and strain or injury by (strains). Despite emerging slowly, office and clerical, lower back, and strains continue to represent a relatively small share of claims in excess of $2M at ultimate.
- Burn and electric shock mega claims have the fastest emergence. Almost all burn and electric shock mega claims are recognized within the first 18 months of policy inception.
- Claims take longer to exceed the $2M threshold than higher thresholds. Based on historic emergence, around 40% of mega claims reach the $2M threshold by 18 months from policy inception and 82% of mega claims reach that threshold by 126 months from policy inception. Emergence patterns are similar at the $3M, $5M, and $10M thresholds. Emergence is speeding up across all mega claims thresholds.
- Since 2017, the share of reported loss over $2Mat 18 months after policy inception has increased. This is consistent with both faster emergence of mega claims and an increase in the relative frequency of mega claims. The share has increased across all industry groups.
- As in the rest of the country, the ultimate frequency of claims greater than $2M from construction workers is increasing in California. This is concentrated in claims from falls and slips. The share of loss greater than $2M has also increased for fall and slip claims.
Read the complete report to learn more.
Save the Date: Thursday, January 16, 2025
Several independent bureaus and NCCI will host an insightful webinar on information derived from this collaborative study. Register Now .

COMPLETE REPORT

RELATED CONTENT

CONNECT WITH US
We use cookies on this site to enhance your user experience. By continuing to use this site you are giving us your consent to place cookies on your device.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 11 December 2024
‘Getting paid to review is justice’: journal pays peer reviewers in cryptocurrency
You can also search for this author in PubMed Google Scholar
An experimental journal is paying peer reviewers the equivalent of US$150 per review in a specially developed cryptocurrency. The publication is hosted on a platform aiming to make science more open and efficient, and rewards users with a token called ResearchCoin for engaging with content.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
doi: https://doi.org/10.1038/d41586-024-04027-4
Reprints and permissions
Related Articles

- Peer review
Apply the legal ‘true malice’ principle to protect research-misconduct sleuths
Correspondence 30 DEC 24

Can novelty scores on papers shift the power dynamics in scientific publishing?
Nature Index 20 DEC 24
Pioneering journal eLife faces major test after loss of impact factor
News 18 DEC 24

‘Doing good science is hard’: retraction of high-profile reproducibility study prompts soul-searching
News 14 OCT 24
Group Leader in Molecular Infection Medicine
We are looking for an excellent young researcher to become a Group Leader for innovative research in molecular infection medicine.
Umeå, Sweden
Umeå University
Faculty position
Pig Genetics in Phenomics, Gene Cloning, Pig biotech breeding. Animal Biobank in Cryobiology, Biogeography, Developmental Biology, Bioinformatics.
Institute of Animal Science, Chinese Academy of Agricultural Sciences
Global recruitment of Principal Investigator
The principal investigators (PI) is responsible to manage research teams focused on innovative drug R&D.
Beijing, China
Sinovac Biotech Ltd.
Researcher/Group leader in wood biology
We are seeking a new group leader (principal investigator) in the field of wood biology at Umeå Plant Science Centre (UPSC).
Umeå (Kommun), Västerbotten (SE)
Swedish University of Agricultural Sciences
Faculty Positions, Aging and Neurodegeneration, Westlake Laboratory of Life Sciences and Biomedicine
Applicants with expertise in aging and neurodegeneration and related areas are particularly encouraged to apply.
Hangzhou, Zhejiang, China
Westlake Laboratory of Life Sciences and Biomedicine (WLLSB)
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Energy compensation and metabolic adaptation: "The Biggest Loser" study reinterpreted
Affiliation.
- 1 Laboratory of Biological Modeling, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland, USA.
- PMID: 34816627
- DOI: 10.1002/oby.23308
"The Biggest Loser" weight-loss competition offered a unique opportunity to investigate human energy metabolism and body composition before, during, and after an extreme lifestyle intervention. Here, I reinterpret the results of "The Biggest Loser" study in the context of a constrained model of human energy expenditure. Specifically, "The Biggest Loser" contestants engaged in large, sustained increases in physical activity that may have caused compensatory metabolic adaptations to substantially decrease resting metabolic rate and thereby minimize changes in total energy expenditure. This interpretation helps explain why the magnitude of persistent metabolic adaptation was largest in contestants with the greatest increases in sustained physical activity and why weight-loss interventions involving lower levels of physical activity have not measured similarly large metabolic adaptations. Additional longitudinal studies quantifying the interrelationships between various components of energy expenditure and energy intake are needed to better understand the dynamics of human body weight regulation.
Published 2021. This article is a U.S. Government work and is in the public domain in the USA.
Publication types
- Research Support, N.I.H., Intramural
- Body Composition* / physiology
- Body Weight / physiology
- Energy Intake
- Energy Metabolism / physiology
- Weight Loss* / physiology
Grants and funding
- 1-ZIA-DK013037/DK/NIDDK NIH HHS/United States
Sopra Steria Reveals a Study on Young Tech Graduates’ Career and HR Expectations
Paris, September 12, 2024 – Sopra Steria, a major player in the European tech sector, presents a study on the aspirations of recent graduates from engineering, IT, and STEM fields across Europe and India. The study confirms a strong interest in breakthrough technologies (AI, quantum computing) and those serving the greater good (developed with a Green IT approach). Policies promoting work-life balance are highly sought after.
Regarding the international panel, key findings include:
- The technologies preferred by young graduates are related to Quantum Computing (60%), followed by Green IT (54%) and Cloud (53%).
- The top three factors influencing job choice are compensation (47%), career development opportunities (36%), and work-life balance (36%).
Louis-Maxime Nègre, Director of Human Resources at Sopra Steria Group, commented : “The results of this study illustrate the growing importance of disruptive technologies and societal values in the aspirations of young tech talent. At Sopra Steria, we firmly believe that technological innovation must be aligned with its contribution to the greater good. This is why we advocate for responsible artificial intelligence and integrate Green IT into our value chain while offering tailored career paths for our talent. Our mission is to create high-performing, sustainable, and impactful digital solutions".
Green IT Leads in the International Panel
In 2024, 60% of young graduates identified quantum computing as the most attractive field, followed closely by Green IT (54%) and cloud computing (53%). However, in France, AI remains the most popular, with 55% of graduates aspiring to work in this domain.
Louis-Maxime Nègre added : “These insights reveal an interesting trend: the appeal of a technology seems closely tied to its position on the Gartner hype curve. Emerging technologies captivate more, at least for a first job, than those that are already in the industrialization phase. For a company like Sopra Steria, this underscores the necessity of maintaining a strong commitment to innovation”.
Compensation Is the Top Criterion for 47% of Graduates
Beyond their technological preferences, tech graduates express significant expectations toward their future employers, particularly in HR developments. The top three priorities identified are:
- Compensation, as the primary criterion by 47% of graduates.
- Work-life balance, prioritised by 36%.
- Career opportunities, also highlighted by 36% of respondents globally.
Once on the job, HR expectations shift slightly. Work-life balance remains the preferred HR offering for young graduates (53% of respondents), ahead of career development programs (48%) and training (43%). Regarding work-life balance solutions, several measures rank among the top choices for young graduates:
- Home office is widely favored — 2 or 3 days a week by 85% of respondents. This intriguing figure nuances the emerging trends of a full return to the office while excluding the norm of 100% remote work.
- Freelancing comes a close second (81%). The rise of freelancing as a preferred option among young tech graduates reflects their quest for flexibility and control over their work-life balance.
Sopra Steria: Ambitious Programs Supporting Innovation and Talent
Anticipating these trends, Sopra Steria has implemented several programs tailored to the expectations of young tech talent, combining innovation, cutting-edge technologies, and responsibility to design and execute major projects serving European citizens.
- A Comprehensive AI Program
The Group has rolled out a program dedicated to artificial intelligence for its 52,000 employees to integrate this technology into all activities where it can make a meaningful impact. This program aims both to train all teams in the use of AI and to embed this technology in all relevant offerings of the Group.
- Developing Solutions with a Green IT Approach
Deeply committed to sustainable digital practices, Sopra Steria also supports its clients in their ecological transition by delivering high-performance solutions developed with a Green IT approach. This approach incorporates solutions designed to reduce the environmental impact of digital activities while balancing economic performance with ecological responsibility.
- Supporting Talent
Sopra Steria offers personalized career development opportunities, enabling talent to upskill in diverse sectors such as industry, banking, and aerospace. These pathways support individual ambitions while addressing current technological and societal challenges.
Leveraging its technological expertise and commitment to sustainable digital practices, the Group strengthens Europe’s digital sovereignty while addressing the strategic challenges of key sectors. This balance of performance, innovation, and responsible impact reflects its ambition to shape a digital future that is both efficient and ethical.
Digest of the study (Full report upon request)
Download the PDF version
All releases
Contact our media department
/aurélien-flaugnatti.jpg?sfvrsn=173c7fdb_5)

IMAGES
COMMENTS
A clinical study with compensation focuses on paying individual for participating in their trials. This helps study teams to more quickly drive interest around a trial, and also helps to ensure that clinical trial participants subsist through the entire trial.
Compensation to participate in research should be ethically appropriate, just, and fair. While protocols may differ in complexity and subject population, investigators must make reasonable judgments about how compensation might potentially affect enrollment and ongoing participation.
Oct 12, 2022 · Under the right circumstances, with the necessary personal attributes, it is possible for you to be paid thousands of dollars to participate in clinical medical trials. You may be asked to take new medications. You may be tested for allergies. You may be scrutinized by new medical technology.
Jul 6, 2021 · Researchers who would like to offer teachers compensation should conduct the study during the teachers’ personal time. Alternatively, if your research activities must fall during class time, consider providing teachers with non-monetary compensation (such as school supplies) or donations.
Aug 15, 2024 · A paid study is a research project in which the entity overseeing the project offers monetary compensation to the participants. Paying participants to enroll in the study allows the research organization to gather a large enough sample size to yield meaningful results.
Apr 23, 2023 · Understanding the current state of and trends in empirical research on employee compensation and performance (our first task), and key findings on PFP specifically (our second task) is of...
As compensation is an expression of gratitude by researchers for their participants, it is not a benefit of participation, and it must be fair and equitable. However, offering compensation is not required or expected.
compensation of research subjects This guidance document is intended for investigators planning to provide compensation (monetary or non-monetary) to subjects for participation in research.
The apparent widespread use of research compensation has in some respects shifted the debate from whether payment should be used to how it should be used to ensure that research compensation practices align with ethical standards and incorporate an equity lens (Collins et al., 2017).
Compensation is a predetermined amount defined within the project for time, effort, inconvenience and general expense to participate in a research study. All BMC research accounts involving payment/reimbursement to research participants and BU research accounts that utilize the BMC Cashier’s Office for issuing payments to participants.
Apr 12, 2021 · Compensation increases recruitment, allowing studies to be completed efficiently, saving time, money, and staff resources. It also recognizes participants for their contribution to advancing medical science.
6 Must-Read Articles on Payments to Research Participants Clinical research is a field continuously changing due to new regulations, trends, strategies and so on. With so much happening in the industry, compensation to research volunteers isn’t always the hottest topic.
If you qualify, you will receive financial compensation of up to $1350. You will also receive all study-related care and services. Please contact us today
Jul 21, 2018 · In clinical research studies, it is not uncommon for monetary compensation to be provided to research participants; as reimbursement for study-related expenses, as compensation for time and effort, and even as incentive payments to encourage enrollment.
Background/aims: Financial compensation for research participation is a major focus of ethical concern regarding human subject recruitment. Phase I trials are sometimes considered to be a lucrative source of income for healthy volunteers, encouraging some people to become "professional guinea pigs."
Research on compensation and employee benefits has enjoyed a long and rich history.
Nov 9, 2020 · We describe experiences of conducting a community-based study of air pollution in southern Malawi incorporating ethnographic, participatory and air quality monitoring elements. Decisions surrounding participant compensation evolved in response to changing circumstances in the field.
May 3, 2010 · To examine whether investigator and IRB recommendations for payment for different types of studies align with actual costs of participation, we calculated participant costs for hypothetical research studies and compared them to recommendations for payment.
Dec 19, 2013 · A study was conducted in Zimbabwe to investigate views and expectations of various stakeholders on study participation incentives, compensation and reimbursement issues.
Dec 12, 2024 · The Workers’ Compensation Insurance Rating Bureau of California (WCIRB), in collaboration with nine other workers’ compensation rating bureaus, has jointly released Countrywide Mega Claims: Accident Years 2001–2021.This study is an update of a 2020 analysis produced in response to stakeholder concerns about the relative frequency and cost of mega claims.
An Executive Compensation Study for CEO position cost $6,500 for members/$6,975 for non-members. In the coming months, Executive Compensation Studies will be available for the following positions: COO, CFO, CFOO, the chief lobbyist, chief communication officer, chief marketing officer and the chief legal position.
Dec 9, 2024 · 5 Most prior studies estimate taxable income based on publicly disclosed financial accounting information. There are a few exceptions, including Mills (Citation 1998) and Graham and Mills (Citation 2008), which do not examine contractual use of taxable income.Mills (Citation 1998) utilizes Internal Revenue Service (IRS) data to measure book-tax differences and investigates the association ...
Dec 12, 2024 · Data for this study were collected as part of a national study of variations in care and outcomes of care for patients with lung or colorectal cancer undertaken by the CanCORS Consortium. 10 CanCORS examined care delivered to population-based cohorts totaling more than 10,000 patients initially diagnosed with lung or colorectal cancer in 2003 ...
Feb 27, 2023 · Accordingly, research on compensation is scientifically and practically important and has been published in the pages of Personnel Psychology (PP) from its inception.
Jun 28, 2024 · The purpose of this study is to understand how the current compensation system affects the balance and know that compensation must be done in accordance with the hard work of employees. Compensation will also affect employee performance, meaning that appropriate compensation will make employees more motivated and willing to work harder ...
Dec 12, 2024 · The study analyzes the emergence of mega claims, defined as claims with incurred loss at 2022 cost levels of at least $2M, unless a different threshold of $3M, $5M or $10M is specified. The updated analysis includes more granular industry group and claim characteristics for claims above $2M and calculates frequency relative to premium and ...
Dec 11, 2024 · Authors can also earn ResearchCoin as a reward for using good research practices, such as preregistering studies and sharing data openly. Users of ResearchHub can already earn ResearchCoin for ...
Here, I reinterpret the results of "The Biggest Loser" study in the context of a constrained model of human energy expenditure. Specifically, "The Biggest Loser" contestants engaged in large, sustained increases in physical activity that may have caused compensatory metabolic adaptations to substantially decrease resting metabolic rate and ...
Dec 11, 2024 · The top three factors influencing job choice are compensation (47%), career development opportunities (36%), and work-life balance (36%). Louis-Maxime Nègre, Director of Human Resources at Sopra Steria Group, commented : “The results of this study illustrate the growing importance of disruptive technologies and societal values in the ...