- For Teachers

- Everyday Activities
- Experiments


Lemon Batteries
Can you get power from a lemon?
Batteries consist of two different metals suspended in an acidic solution.
Is it possible to use the acid in a lemon to power a light? Try it to find out!
Watch the video on YouTube: QYZE-SrpoJ4
You Will Need
4 or more large, fresh, juicy lemons or other citrus fruits
A kitchen knife
4 or more zinc electrodes You can find galvanized washers or roofing nails at most hardware stores, or you can purchase either zinc or magnesium wire online
4 copper electrodes Copper-coated pennies work, or you can find bare copper wire or copper plumbing fittings at most hardware stores
1 light-emitting diode (LED) component We recommend a red LED because they typically need lower voltages to glow than other colors, but many colors will work. The best LEDs for this experiment are designed to glow with a low current such as this set from Amazon , or you can purchase a lesser quantity from Mouser electronics .
6 or more lead wires with alligator clips. Such as this set available on Amazon
Multimeter (optional)
Materials & Directions PDF
NOTE: Some retailers also sell lemon battery kits that can include a buzzer or a low voltage clock. These instructions assume you are using your lemon battery to make an LED glow.
- Ask your scientist to create a testable question.
- Carefully clean your zinc and copper electrodes to remove any dirt or grease. (Careful not to scrub all of the zinc coating off the galvanized washers or nails.)
- Roll one lemon on a hard surface while pushing down to break the cell walls and loosen up the juice inside. The sour (acidic) juice is needed for the chemical reaction that you are about to start.
- Place the lemon on its side on a plate and have an adult carefully use the kitchen knife to make a 2 small cuts in the top of the lemon. Make each cut about two centimeters long, one centimeter deep, and about 0.5-1 centimeters apart. (To conserve lemons, you can cut 1 lemon in half, and put the electrodes in either the cut side or the rind side.)
- Insert one zinc electrode deep into one of the cuts, and one copper electrode deep into the other. Leave some sticking out so you can connect your wires to them. You have now made a lemon cell!
- Set the multimeter to measure DC voltage (V with a straight line). If it has scale options, set it to measure 2000 millivolts (2000m).
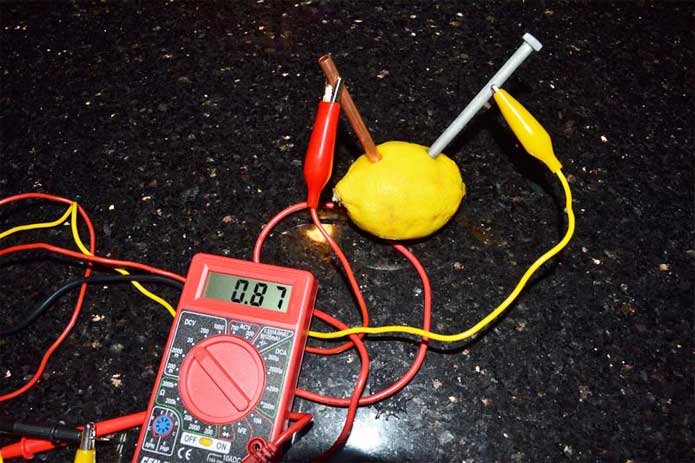
- Hook two alligator clips from your leads to the two electrodes. Attach red lead of the multimeter (+) to the copper electrode, and the black lead of the multimeter (-) to the zinc electrode.
- Measure the voltage from your lemon cell. The average lemon cell should read about 0.9–1.0 volts.
- Now set the multimeter to measure current (A with a squiggly line: mÃ/Ã). If it has scale options, set it to measure 1–20mÃ.
- Measure the current of your lemon cell. It should read a few tenths of a milliampere. Some multimeters are not sensitive enough to measure currents less than one milliampere, in which case you will see 0.0 as the reading.
- A red LED typically needs a voltage of 1.2–1.6 V, so we need more power to light the bulb.
- Follow steps 3-5 to make 3 or 4 more lemon cells.
- (Optional) If you have a multimeter, check each lemon battery to make sure it generates voltage and current.
- Connect the zinc electrode on the first lemon to the copper electrode on the second lemon.
- Connect the zinc electrode on the second lemon to the copper electrode on the third lemon.
- Repeat if using more lemons. This type of connection is called a series circuit , and provides one path through which electricity can flow.
- Gently bend the lead wires of the LED apart from each other.
- Connect a lead wire from the copper electrode of the first lemon cell to the longer lead wire from the LED.
- Connect a lead wire from the zinc electrode of the third cell to the shorter wire of the LED.
- Turn down the room lights to see if your LED is glowing! If it isn’t, see the troubleshooting tips below.
Troubleshooting your lemon battery:
- Ensure the electrodes are not touching inside lemon.
- Ensure the alligator clips on the test lead wires are not touching each other where you connect them to the LED.
- The wires from one lemon to the other have to be connected from zinc to copper in order for the electricity to flow.
- Is it an old lemon? The lemon needs to be juicy inside.
- Do you need to add more lemons?
- Is your LED broken? Or does it require a higher voltage to work?
- The quality of the copper and zinc can be problematic. Pennies are rarely pure copper. Try substituting a length of 14 gauge copper wire (common house wire). Experiment with different lengths and configurations of electrodes. Other sources of zinc and copper may be found in the plumbing department of a hardware store.
Discovery Questions
Beginning the experiment, during the experiment, after the experiment, how it works.
Electrochemical cells, also called batteries, require three things—two electrodes and one electrolyte. One of the electrodes has to have a stronger desire for electrons than the other—in chemistry we say that it has a higher electronegativity . The electrode that wants the electrons more is called the cathode , and the one that gives up electrons is electropositive and is called the anode .
Copper likes having electrons more than zinc so it’s more electronegative and is the cathode, leaving zinc to be the anode. An electrolyte is a solution that conducts electricity. The lemon provides citric acid, and acids contain ions which conduct electricity, making the lemon our electrolyte .
When zinc is exposed to the acid in the lemon juice, the acid oxidizes—or removes electrons from the zinc. The resulting positively charged zinc ions move into the lemon juice, and the resulting electrons collect in the zinc metal. They then rush across the wire into the copper which wants electrons more than zinc. Those electrons, now in the copper, pull a couple of protons or hydrogen ions out of the acid and reduce them, adding electrons, which creates hydrogen gas. If we could see inside the lemon, we might be able to see very very tiny bubbles of hydrogen gas forming on the copper electrode. In summary, the electricity is not coming from the lemon by itself, but from the chemical reaction resulting from the differences in electronegativities between zinc and copper.
Source: Dr. Christopher Gorski and Jenelle Fortunato, Penn State College of Engineering

Fun Lemon Battery Science Fair Experiment for Kids

Please enable JavaScript

We all heard of making lemonade out of lemons and that when things go wrong, if you know to Think in The Right Way then you can make something good come out of a sour situation. But how can you make a BATTERY out of Lemons?!

Most of us know what Lemons look like; A Lemon is a yellow, oval, thick skinned and fragrant citrus fruit said to originate from India and is commonly grown in the Middle East .

Lemons are a good source of Vitamin C, which is important to boost our immune system in order to fight sickness. Lemons are used in aromatherapy, but they are not just healthy for you, they are also used for culinary purposes; lemon juice, lemon cakes, lemon cookies, a tarty lemon pie, lemon ice cream and in food preparations like Tahini, Hummus, salads and many other dishes in many countries around the world.

However, do you know that the lemon is also used as a natural, non-toxic mosquito repellent and insecticide? Other interesting uses for lemons are as a cleaning agent to remove stains and grease, or to deodorize. Some kids (and even adults) make money from selling lemonade in street stalls, fairs, festivals and local markets.
Lemons can also provide a lot of fun for your kids, even if they hate drinking lemon juice. One of the interesting things you can do with your kids and a few lemons, is a fun lemon battery science fair experiment ! It is an easy, low cost and fun educational activity to do at home or at school. Try this fun lemon science experiment with your kids to teach kids about electrical circuits!
How to Make a Battery from Lemons
Here’s how you can make your own homemade lemon battery that’s strong enough to light a bulb. This fun lemon battery science fair experiment creates what is called a wet cell battery; normal batteries are dry cells.

What you need for the Lemon Battery Science Fair Experiment:
- A penny or a copper coin or a copper strip
- A nail coated in zinc
- A volt meter (optional) to help you understand the voltage produced by the lemon and can measure it.
5 Easy Steps to Make a Homemade Lemon Battery:
1. Polish and rub the zinc coated nail and the penny or copper coin with sandpaper. 2. Without cutting the lemon or breaking its skin, give it a few strong squeezes. Squeezing the lemon releases juices inside the lemon. 3. Make two slits on the top of the lemon, half an inch apart. 4. Insert a penny (or copper coin) into one slit and the zinc coated nail into the other slit. 5. Connect to a volt meter to see if voltage is produced by touching leads of voltmeter to both the penny and the zinc.
TIP: The power from one single lemon will not be strong enough to light a bulb or be used as a battery. You will need to connect several lemons together with metal wires for enough power or electricity to light a bulb from a lemon battery. Minimum two lemons will create a battery but 3 are better. See what happens if you add more lemons. What will the volt meter show? How strong will the bulb glow?
Fun Facts about Lemons
- In India, lemons are used in traditional Siddha Medicine and Ayurveda, due to the lemon’s anti-bacterial properties.
- One of the benefits of Lemon juice is that it is a digestive aid that cleans your liver.
- We all love to drink cold Lemonade on hot summer days. However, mixing sugar with the acidic lemon might not be a good idea for your teeth .
- Lemons contain many healthy substances such as citric acid, calcium, magnesium, vitamin C, bioflavonoids, pectin, and limonene. These substances in lemon promote a healthy immune system and fight infection.
- The minerals found in Lemon juice improve the appearance of your hair, skin and nails.
- Lemons can help to get rid of acne, are useful when you have a fever or flu, can cure corns and calluses, ease eczema, lighten dark spots and blemishes, remove blackheads, freshen your breath, strengthen nails and even whiten your teeth.
- Lemons are green when they aren’t ripe yet, which makes it easy to mistake them with limes. Though lemons and limes are very similar, the easiest way to tell them apart is to look at the shape. A lemon has a shape like a football with stubs at opposite ends, while the lime has a circular or perfect oval shape.

Lemon Battery science Fair experiment Video for Kids
Here’s a great video for kids on how to make a battery from lemons:
Image sources, thanks and references: Lemon Pie image – candacesalima.blogspot.com Lemon Chicken image – theblog.jessikerbakes.com Lemon Tart picture – rawfoodrecipes.com Lemonade image – sciencedaily.com Lemon Bars photo – parade.condenast.com Lemon Butter-cake image with lemon juice glaze – cozycakescottage.com Lemon Salmon Fish picture – healthykidscompany.com
Enjoyed this Easy Science for Kids Lemon Battery Science Fair Experiment ? Find more exciting science fair project ideas and fun science fact for kids , free science worksheets for kids and free online interactive quizzes . For very lengthy info about lemons visit Wikipedia.

Lemon and Potato Battery Experiment
Learn how to generate electricity from common fruit or vegetables.
Posted by Admin / in Energy & Electricity Experiments
Is it possible to produce electricity from common fruit or vegetables? Fruits and vegetables require energy from the sun to grow and produce a harvest. Is it possible that some of the sun's energy is stored in the produce for our use? We know that by eating fruits and vegetables our body can convert this food to energy. Is it possible to directly generate electricity from a piece of fruit or a vegetable. This lemon battery and potato battery science experiment tests this theory.
Materials Needed
- Copper strip or rod
- Zinc strip or zinc-coated bolt
- Circuit wire or alligator clips with wire
EXPERIMENT STEPS

Step 1: Cut 2 small slits in the skin of both the lemon and the potato. Make the slits are a few inches apart.

Step 2: Push the copper and zinc strips into the slits in each piece of produce. Make sure the rods do not touch each other.

Step 3: Connect an electrical wire to the end of each metal strip. Alligator clips make this step easy.

Step 4: Measure the voltage drop between the two wires attached to the metal strips on the lemon and the potato. This is the amount of voltage being produced by each piece of produce. Compare the difference in the amount of voltage produced by a lemon and a potato. What do you notice? How long will the fruit and vegetable generate voltage?
Science Learned
The lemon and the potato act like a low-power battery. This experiment shows how a wet cell battery works. Chemicals in the fruit or vegetable create a negative charge in the zinc strip. Electrons move into the zinc strip and travel up the wire attached. The electrons then travel through the voltmeter which measures the voltage drop and end up in the copper strip which becomes the positive end of the circuit. Pardon the pun, but from this experiment we can say that it is possible to "produce electricity".
Oberlin College: Demonstration of lemon battery powering a buzzer .
U.S. Dept. of Energy: Calculating Lemon Battery Power Q&A
- About the author
- Back to Experiment
Please select the social network you want to share this page with:
We like you too :)
Thanks for taking time to give us feedback!
- Energy & Electricity experiments
- science experiments for kids
- lemon and potato battery experiment
- lemon battery experiment
- fruit battery
- potato battery experiment/a>
- vegetable battery

posted by Admin
- previous experiment
- next experiment

How to Make a Simple Battery
in Energy and Electricity Experiments
Make a simple battery using coins and other common items.

Solar Energy Experiment for Kids
Teach kids how light is used to generate electricity in this solar energy experiment.

Beginner Electronics Experiment For Kids
This experiment is a good starting point for kids to begin learning about electronics.

LED Solar Circuit Experiment
Learn how to make an electrical circuit to power an LED using solar power.

How to Make an Electromagnet
Test the relationship between electricity and magnetism by making an electromagnet.

Power a Light with Static Electricity
Use static electricity to power a light bulb!
